Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a.n_{Zn}=\dfrac{13}{65}=0,2mol\\ Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
0,2 0,4 0,2 0,2
\(V_{H_2}=0,2.24,79=4,958l\\ b.m_{ZnCl_2}=0,2.136=27,2g\\ c.m_{dd\left(sau.pư\right)}=\dfrac{0,4.36,5}{7,3}\cdot100+13-0,2.2=212,6g\\ C_{\%ZnCl_2}=\dfrac{27,2}{212,6}\cdot100=12,79\%\)

\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\\Zn+2HCl\rightarrow ZnCl_2+H_2\\ n_{HCl}=0,2.2=0,4\left(mol\right)\\ m_{ddHCl}=\dfrac{0,4.36,5.100}{10}=146\left(g\right)\\ n_{H_2}=n_{ZnCl_2}=n_{Zn}=0,2\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,2.22,4=4,48\left(l\right)\\ C\%_{ddZnCl_2}=\dfrac{136.0,2}{13+146-0,2.2}.100\approx17,15\%\)
\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
PTHH: Zn + 2HCl → ZnCl2 + H2
Mol: 0,2 0,4 0,2 0,2
\(V_{H_2}=0,2.22,4=4,48\left(l\right)\)
\(m_{ddHCl}=\dfrac{0,4.36,5.100}{10}=146\left(g\right)\)
mdd sau pứ = 13+146-0,2.2 = 158,6 (g)
\(C\%_{ddZnCl_2}=\dfrac{0,2.136.100\%}{158,6}=17,15\%\)

Bài 2:
\(PTHH:Fe+2HCl\rightarrow FeCl_2+H_2\\ n_{H_2}=\dfrac{10,08}{22,4}=0,45\left(mol\right)\\ n_{Fe}=n_{H_2}=0,45\left(mol\right);n_{HCl}=2.0,45=0,9\left(mol\right)\\ a,m_{Fe}=0,45.56=25,2\left(g\right)\\ b,C_{MddHCl}=\dfrac{0,9}{0,15}=6\left(M\right)\)

Gọi x, y lần lượt là số mol của Zn và Fe
Ta có: \(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
a. PTHH:
Zn + 2HCl ---> ZnCl2 + H2 (1)
Fe + 2HCl ---> FeCl2 + H2 (2)
Theo PT(1): \(n_{H_2}=n_{Zn}=x\left(mol\right)\)
Theo PT(2): \(n_{H_2}=n_{Fe}=y\left(mol\right)\)
=> x + y = 0,3 (*)
Theo đề, ta có: 65x + 56y = 17,7 (**)
Từ (*) và (**), ta có HPT:
\(\left\{{}\begin{matrix}x+y=0,3\\65x+56y=17,7\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
=> \(m_{Zn}=0,1.65=6,5\left(g\right)\)
=> \(\%_{m_{Zn}}=\dfrac{6,5}{17,7}.100\%=36,72\%\)
\(\%_{m_{Fe}}=100\%-36,72\%=63,28\%\)
b. Ta có: \(n_{hh_{Zn,Fe}}=0,1+0,2=0,3\left(mol\right)\)
Theo PT(1, 2): \(n_{HCl}=2.n_{hh}=2.0,3=0,6\left(mol\right)\)
=> \(m_{HCl}=0,6.36,5=21,9\left(g\right)\)
=> \(C_{\%_{HCl}}=\dfrac{21,9}{200}.100\%=10,95\%\)

a)
$Fe + 2HCl \to FeCl_2 + H_2$
Theo PTHH :
$n_{Fe} = n_{H_2} = \dfrac{11,2}{22,4} = 0,5(mol)$
$A = 0,5.56 = 28(gam)$
b) $n_{HCl} = 2n_{H_2} = 1(mol)$
$m_{HCl} = 1.36,5 = 36,5(gam)$
c) $m_{dd\ HCl} = 36,5 : 20\% = 182,5(gam)$
$m_{dd\ sau\ pư} = 28 + 182,5 - 0,5.2 = 209,5(gam)$
$C\%_{FeCl_2} = \dfrac{0,5.127}{209,5}.100\% = 30,3\%$

\(n_{H_2}=\dfrac{2.24}{22.4}=0.1\left(mol\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(0.1.......0.2......................0.1\)
Chất rắn X : Cu
\(m_{Zn}=0.1\cdot65=6.5\left(g\right)\Rightarrow m_{Cu}=19.3-6.5=12.8\left(g\right)\)
\(n_{Cu}=\dfrac{12.8}{64}=0.2\left(mol\right)\)
\(C_{M_{HCl}}=\dfrac{0.2}{0.2}=1\left(M\right)\)
\(2Cu+O_2\underrightarrow{^{^{t^o}}}2CuO\)
\(0.2........0.1\)
\(m_{tăng}=m_{O_2}=0.1\cdot32=3.2\left(g\right)\)
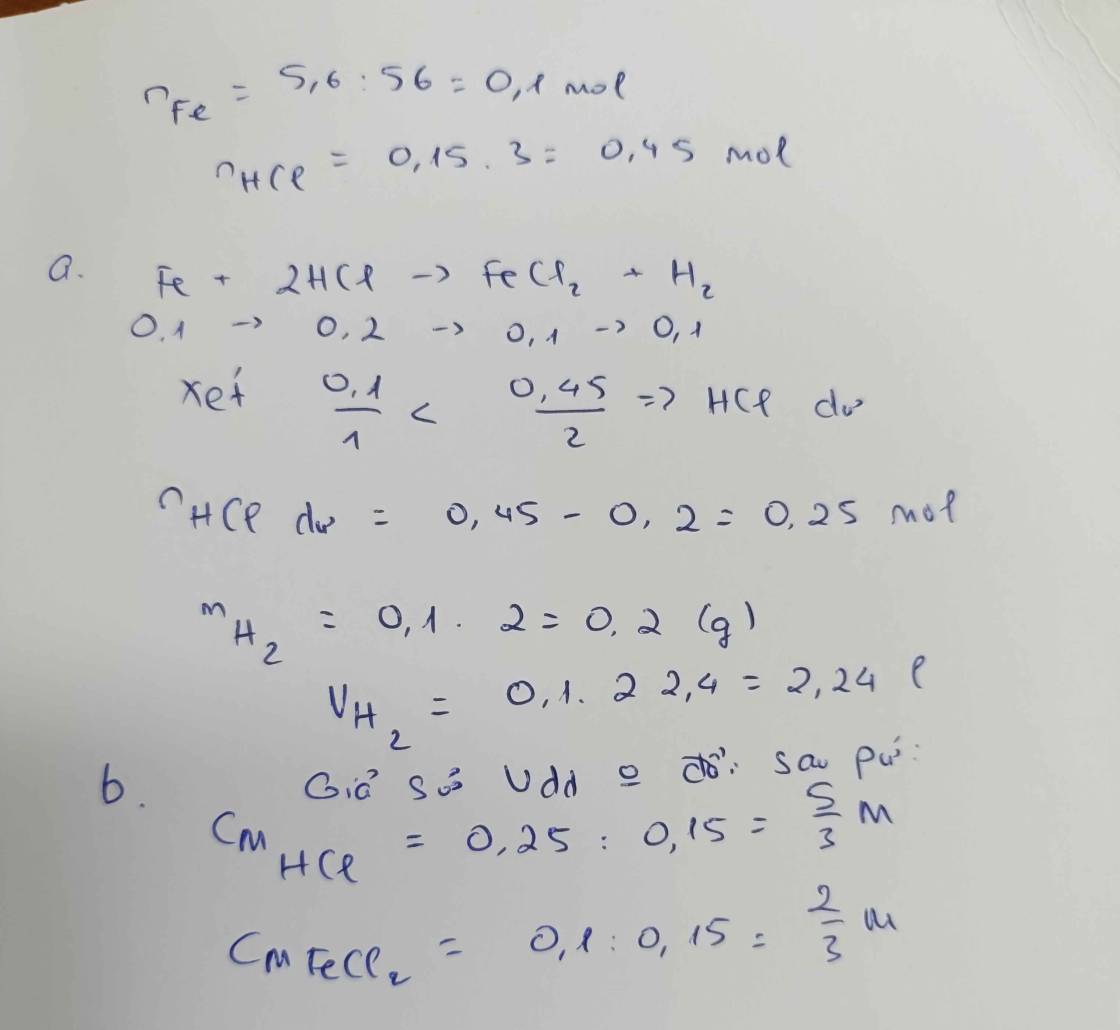

\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
a, Theo PT: \(n_{H_2}=n_{Zn}=0,1\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,1.24,79=2,479\left(l\right)\)
b, \(n_{HCl}=2n_{Zn}=0,2\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,2}{0,15}=\dfrac{4}{3}\left(M\right)\)