Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{P_2O_5}=\dfrac{99,4}{142}=0,7\left(mol\right)\)
\(P_2O_5+3H_2O\rightarrow2H_3PO_4\)
0,7 2,1 1,4
a, \(m_{H_3PO_4}=1,4.98=137,2\left(g\right)\)
\(m_{ddH_3PO_4}=99,4+500=599,4\left(g\right)\)
Kl nước trong dd A :
\(m_{H_2O}=599,4-137,2=462,2\left(g\right)\)
\(b,C\%_{H_3PO_4}=\dfrac{137,2}{599,4}.100\%\approx22,89\%\)
\(c,C_M=\dfrac{n}{V}=\dfrac{1,4}{0,5}=2,8M\)

\(a,C\%_{KOH}=\dfrac{28}{140}.100\%=20\%\\ b,C\%_{KOH}=\dfrac{80}{80+320}.100\%=20\%\)

a.\(n_{NaOH}=\dfrac{8}{40}=0,2mol\)
\(V_{dd}=\dfrac{120}{1,2}=100ml=0,1l\)
\(C_{M_{NaOH}}=\dfrac{0,2}{0,1}=2M\)
b.\(n_{NaOH}=\dfrac{21,6}{40}=0,54mol\)
\(V_{dd}=\dfrac{180}{1,2}=150ml=0,15l\)
\(C_{M_{NaOH}}=\dfrac{0,54}{0,15}=3,6M\)

\(C\%=\dfrac{30}{170}.100\%=17,647\%\)
\(V_{\text{dd}}=\left(30+170\right)1,1=220ml\)
\(n_{NaCl}=\dfrac{30}{58,5}=0,513mol\)
\(C_M=\dfrac{0,513}{0,22}=0,696M\)
\(C\%_{NaCl}=\dfrac{30}{170+30}.100\%=15\%\\ C_M=C\%.\dfrac{10D}{M}=10.\dfrac{10.1,1}{58,5}=1,88M\)

\(a,C_{M\left(NaOH\right)}=\dfrac{0,3}{0,5}=0,6M\\ b,n_{NaOH}=\dfrac{24}{40}=0,6\left(mol\right)\\ C_{M\left(NaOH\right)}=\dfrac{0,6}{0,4}=1,5M\)

a, \(n_{P_2O_5}=\dfrac{21,3}{142}=0,15\left(mol\right)\)
PT: \(P_2O_5+3H_2O\rightarrow2H_3PO_4\)
Theo PT: \(n_{H_3PO_4}=2n_{P_2O_5}=0,3\left(mol\right)\)
\(\Rightarrow m_{H_3PO_4}=0,3.98=29,4\left(g\right)\)
b, m dd sau pư = 21,3 + 300 = 321,3 (g)
\(\Rightarrow C\%_{H_3PO_4}=\dfrac{29,4}{321,3}.100\%\approx9,15\%\)

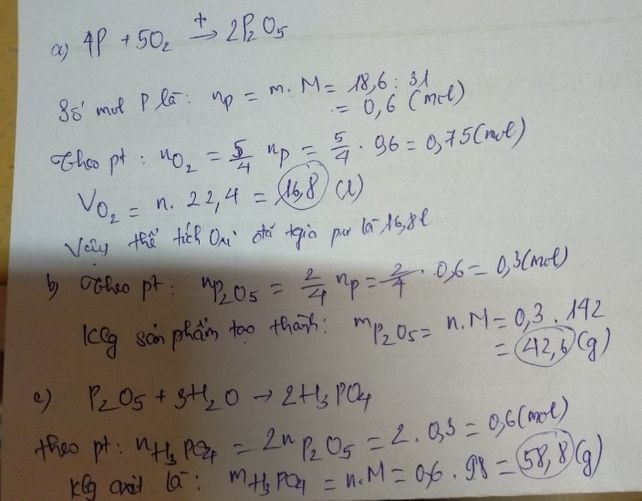
 Bạn tham khảo cách làm nhé! cho mik 1 like nha
Bạn tham khảo cách làm nhé! cho mik 1 like nha
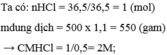
Ta có: \(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\)
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
____0,2__________0,1 (mol)
\(P_2O_5+3H_2O\rightarrow2H_3PO_4\)
0,1_________________0,2 (mol)
\(\Rightarrow C_{M_{H_3PO_4}}=\dfrac{0,2}{0,4}=0,5\left(M\right)\)
Ta có: \(m_{ddH_3PO_4}=400.1,15=460\left(g\right)\)
\(\Rightarrow C\%_{H_3PO_4}=\dfrac{0,2.98}{460}.100\%\approx4,26\%\)