
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(n_{H^+}=2.0,4.0,05=0,04mol\\ n_{OH^-}=2.0,1.0,175=0,035mol\\ pH=-log\left[H^+\right]=-log\left(\dfrac{0,04-0,035}{0,4+0,1}\right)=2\)

Ta có: \(n_{H^+}=n_{HCl}=0,04.0,75=0,03\left(mol\right)\)
\(n_{OH^-}=2n_{Ba\left(OH\right)_2}+n_{KOH}=2.0,16.0,08+0,16.0,04=0,032\left(mol\right)\)
PT: \(H^++OH^-\rightarrow H_2O\)
____0,03______0,03 (mol)
\(\Rightarrow n_{OH^-\left(dư\right)}=0,032-0,03=0,002\left(mol\right)\)
\(\Rightarrow\left[OH^-\right]=\dfrac{0,002}{0,04+0,16}=0,01\left(M\right)\)
\(\Rightarrow\left[H^+\right]=\dfrac{10^{-14}}{0,01}=10^{-12}\left(M\right)\)
\(\Rightarrow pH=-log\left[H^+\right]=12\)

Ta có
\(\text{nHCl=0,2.0,1=0,02(mol)}\)
\(\text{nH2SO4=0,2.0,05=0,01(mol)}\)
2HCl+Ba(OH)2\(\rightarrow\)BaCl2+2H2O
H2SO4+Ba(OH)2\(\rightarrow\)BaSO4+2H2O
Ta có pH=13\(\rightarrow\)Ba(OH)2 dư
pH=13\(\rightarrow\)pOH=1\(\Rightarrow\)CM[OH-]=0,1(M)
\(\rightarrow\)CMBa(OH)2 dư=0,05(M)
\(\text{nBa(OH)2 dư=0,05.0,5=0,025(mol)}\)
\(\text{m=0,01.233=2,33(g)}\)
nBa(OH)2=0,025+0,02/2+0,01=0,045(mol)
\(\rightarrow\)a=\(\frac{0,045}{0,3}\)=0,15(M)

Câu 1:
PT ion: \(H^++OH^-\rightarrow H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{OH^-}=0,6\cdot0,4+0,6\cdot0,3\cdot2=0,6\left(mol\right)\\n_{H^+}=0,2\cdot2,6=0,52\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\) H+ hết, OH- còn dư \(\Rightarrow n_{OH^-\left(dư\right)}=0,08\left(mol\right)\)
\(\Rightarrow\left[OH^-\right]=\dfrac{0,08}{0,6+0,2}=0,1\left(M\right)\) \(\Rightarrow pH=14+log\left(0,1\right)=13\)
Bài 2:
PT ion: \(H^++OH^-\rightarrow H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{OH^-}=0,3\cdot1,6=0,48\left(mol\right)\\n_{H^+}=0,2\cdot1\cdot2+0,2\cdot2=0,8\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\) OH- hết, H+ còn dư \(\Rightarrow n_{H^+\left(dư\right)}=0,32\left(mol\right)\)
\(\Rightarrow\left[H^+\right]=\dfrac{0,32}{0,2+0,3}=0,64\left(M\right)\) \(\Rightarrow pH=-log\left(0,64\right)\approx0,19\)
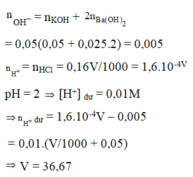
\(n_{Ba\left(OH\right)_2}=\dfrac{0.548}{171}\left(mol\right)\)
\(C_{M_{Ba\left(OH\right)_2}}=\dfrac{\dfrac{0.548}{171}}{0.8}=0.004\left(M\right)\)
\(pH=14+log\left[OH^-\right]=14+log\left(0.004\cdot2\right)=11.9\)