Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{hh}=6,72:22,4=0,3mol\\ C_2H_2+2Br_2->C_2H_2Br_4\\ C_2H_4+Br_2->C_2H_2Br_2\\ n_{Br_2}=0,4mol\\ n_{C_2H_2}=a;n_{C_2H_4}=b\\ a+b=0,3\\ 2a+b=0,4\\ a=0,2;b=0,1\\ \%V_{C_2H_2}=\dfrac{0,2}{0,3}.100\%=66,67\%\\ \%V_{C_2H_4}=33,33\%\)
1/2 hỗn hợp có 0,1 mol C2H2 và 0,05mol C2H4
\(BT.C:n_{CO_2}=2n_{C_2H_2}+2n_{C_2H_4}=0,3mol\\ n_{CaCO_3}=n_{CO_2}=0,3\\ m_{KT}=0,3.100=30g\)

\(n_{hh}=\dfrac{6,72}{22,4}=0,3mol\)
\(n_{C_2H_4Br_2}=\dfrac{16}{188}=\dfrac{4}{47}mol\)
\(\Rightarrow n_{etilen}=\dfrac{4}{47}mol\)
\(\Rightarrow n_{metan}=0,3-\dfrac{4}{47}=\dfrac{101}{470}mol\)
\(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
\(\%V_{etilen}=\dfrac{\dfrac{4}{47}}{0,3}\cdot100\%=28,37\%\)
\(\%V_{metan}=100\%-28,37\%=71,63\%\)

Cho hỗn hợp qua dung dịch brom dư
\(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
Khí thoát ra là \(CH_4\)
\(CH_4+2O_2\rightarrow^{t^o}CO_2+2H_2O\)
\(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
Ta có:
\(n_{CaCO_3}=\frac{40}{100}=0,4mol=n_{CO_2}=n_{CH_4}\)
\(\rightarrow V_{CH_4}=0,4.22,4=8,96l\)
\(\rightarrow\%V_{CH_4}=\frac{8,96}{13,56}=66\%\rightarrow\%V_{C_2H_4}=34\%\)

\(n_{Br_2}=\dfrac{m_{Br_2}}{M_{Br_2}}=\dfrac{16}{160}=0,1mol\)
\(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
0,1 0,1 ( mol )
\(\%V_{C_2H_4}=\dfrac{0,1.22,4}{16,8}.100=13,33\%\)
\(\%V_{CH_4}=100\%-13,33\%=86,67\%\)

\(n_{Br_2}=\dfrac{4}{160}=0,025\left(mol\right);n_{hh}=\dfrac{2,8}{22,4}=0,125\left(mol\right)\)
PTHH: \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
0,025<-0,125
\(\Rightarrow\left\{{}\begin{matrix}\%V_{C_2H_4}=\dfrac{0,025}{0,125}.100\%=20\%\\\%V_{CH_4}=100\%-20\%=80\%\end{matrix}\right.\)

a.\(m_{Br_2}=8g\)
\(n_{Br_2}=\dfrac{8}{160}=0,05mol\)
\(n_{hh}=\dfrac{4,48}{22,4}=0,2mol\)
\(C_2H_2+2Br_2\rightarrow C_2H_2Br_4\)
0,025 0,05 ( mol )
\(\%V_{C_2H_2}=\dfrac{0,025}{0,2}.100=12,5\%\)
\(\%V_{CH_4}=100\%-12,5\%=87,5\%\)
b.
\(m_{C_2H_2}=0,025.26=0,65g\)
\(m_{CH_4}=\left(0,2-0,025\right).16=2,8g\)
c.
\(2C_2H_2+5O_2\rightarrow\left(t^o\right)4CO_2+2H_2O\)
0,025 0,0625 ( mol )
\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
0,175 0,35 ( mol )
\(V_{kk}=V_{O_2}.5=\left(0,35+0,0625\right).22,4.5=46,2l\)
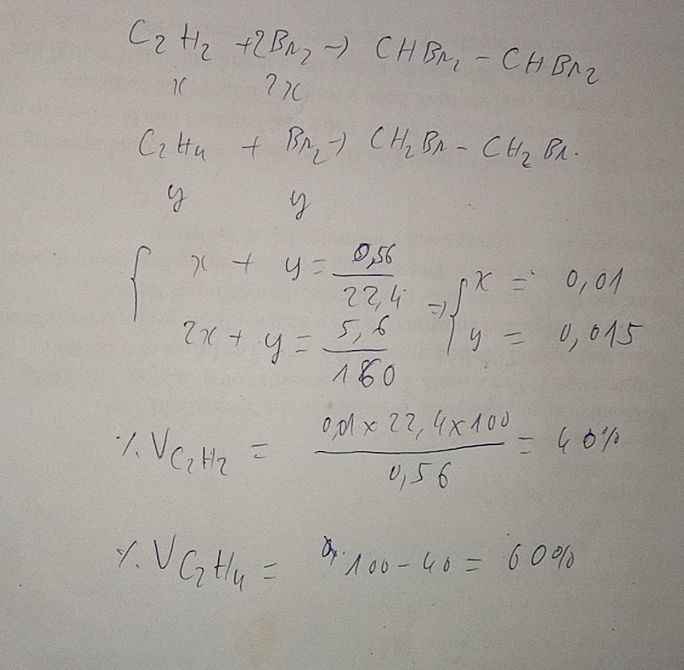
\(a) C_2H_4 + Br_2 \to C_2H_4Br_2\\ n_{C_2H_4} = n_{Br_2} = \dfrac{48}{160} = 0,3(mol)\\ \%V_{C_2H_4} = \dfrac{0,3.22,4}{8,96}.100\% = 75\%\\ \%V_{CH_4} = 100\% -75\% = 25\%\\ b)\)
Khí còn lại : CH4
\(CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + H_2O\\ n_{CO_2} = n_{CH_4} = \dfrac{8,96.25\%}{22,4} = 0,1(mol)\\ m_{CO_2} = 0,1.44 = 4,4(gam)\)