Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH : 2SO2+ O2--> 2SO3 ( điều kiện là nhiệt độ ,xúc tác V2O5);
--> nSO3= nSO2=8/22,4 = 5/14 (mol);
mH2SO4 trong dd = 57,2 * 1,5 * 60%=51,48 (g);
PTHH : SO3+ H2O--> H2SO4;
--> mH2SO4 tạo thành = 5/14 * 98=35(g);
C%dd axit thu được = (51,48+ 35) / (57,2* 1,5+35) =71,59%;
PTHH: 2SO2 + O2 --> 2SO3 (1)
SO3 + H2O --> H2SO4 (2)
Theo PT(1): nSO2=nSO3=8/22,4=5/14 mol
=> mSO3=5/14.80=200/7 g
Theo PT(2): nH2SO4=nSO3=5/14 mol
=> mH2SO4=5/14.98=35g
Mặt khác:
mdd.H2SO4 = 57,2.1,5 = 85,8g
=> mH2SO4 = 85,8.60% = 51,48g
=> Tổng khối lượng H2SO4 = 35 + 51,48 = 86,48g
=> mdd sau p/ứ = 85,8+200/7=4003/35g
=> C% của dd axit thu được = 86,48/4003/35.100%=75,61%

Câu 1:
Khối lượng CaO: 
Số mol CaO:
Pt:
 số mol Ca (OH)2
số mol Ca (OH)2
Vậy khối lượng Ca(OH)2tạo thành: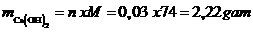
Vậy mct = 2,22 gam
 Mà
Mà 
Vậy nồng độ phần trăm Ca(OH)2: 
Câu 2:
+ Khối lượng riêng  khối lượng dd H2SO4 là
khối lượng dd H2SO4 là 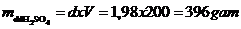
+ 
Số mol CuO: 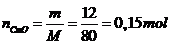
 Pt:
Pt: 

 Khối lượng
Khối lượng 
Vậy khối lượng chất tan: mct = 24 gam
Mà 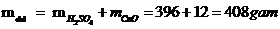
Vậy nồng độ phần trăm: 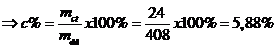

B1: nH2=0,42mol
PTHH: 2M+2nHCl=> 2MCln+nH2
0,84:nmol<-----------0,42mol
=>PTK của M =7,56n/0,84<=> M=9n
ta xét các gtri
n=1=> M=9 loại
n=2=> n=18 loại
n=3=>M=27 nhận
vậy M là Al ( nhôm)
B2: n khí =0,05mol
gọi x,y là số mol của Mg và Zn trong hh:
PTHH: Mg+H2SO4=> MgSO4+H2
x-->x------------->x------>x
Zn+H2SO4=>ZnSO4+H2
y--->y----------->y---->y
theo đề ta có hpt: \(\begin{cases}24x+65y=2,43\\x+y=0,05\end{cases}\)
<=> \(\begin{cases}x=0,02\\y=0,03\end{cases}\)
=> m muối MgSO4=0,02.120=2,4g
m muối ZnSO4=0,03.161=4,83g

1. mH2SO4=98g
C%=98%-3,405%=94,595%
=>mdd sau=mH2SO4/0,94595=103,6g
=>mH2O=103,6-100=3,6
=>nH2O=0,2
=>nO trog oxit=nH2O =0,2
(giai thich: cu 1 mol H2 pu thi lay di 1 mol O trog oxit)
nFe=nH2=0,15
=>nFe:nO=0,15:0,2=3:4
=>Fe3O4.
2. nNa2SO3=0,1
=>nSO2=0,1
nFe2(SO4)3=0,3
vi la hoa 9 nen bat buoc phai viet pthh:
2FexOy+(6x-2y)H2SO4=xFe2(SO4)3+(3x-2y)S...
ti le: 0,3/x=0,1/(3x-2y)
=>x=9x-6y
=>x:y=3:4
=>Fe3O4

a) -Trích mỗi đ 1 ít làm mẫu thử
- Nhỏ vài giọt các dung dịch vào quỳ tím
+ Quỳ tím chuyển sang đỏ : HCl , H2SO4 ( nhóm I )
+ Không đổi màu quỳ tím : Na2SO4 , NaCl ( nhóm II )
- Cho BaCl2 lần lượt vào các đ ở nhóm I , thấy xuất hiện kết tủa trắng thì đó là H2SO4 , còn lại là HCl
BaCl2 + H2SO4 → BaSO4↓ + 2HCl
- Cho Ba(OH)2 vào 2 đ trong nhóm II , thấy xuất hiện kết tủa trắng thì đó là Na2SO4 , còn lại là NaCl
Na2SO4 + Ba(OH)2 → BaSO4↓ + 2NaOH
b) - Trích mỗi chất 1 ít làm mẫu thử
- Cho nước vào 4 mãu thử trên , mẫu thử nào tan tạo thành đ và làm quỳ tím chuyển sang màu xanh là : BaO , K2O , CaO . Không có hiện tượng gì là Al2SO3
CaO + H2O → Ca(OH)2
K2O + H2O → 2KOH
BaO + H2O → Ba(OH)2
- Sục khí SO2 vào 3 dd còn lại , thấy xuất hiện vẫn đục thì chất ban đầu là CaO
Ca(OH)2 + CO2 → CaCO3 + H2O
- Cho H2SO4 vào 2 dd còn lại , tháy xuất hiện kết tủa trắng thì chất ban đầu là BaO , còn lại là K2O
BaO + H2SO4 → BaSO4 + H2
c) - Sụt các khí vào dd nước Br , thấy nước Br bị mất màu thì đó là SO2
SO2 + Br2 + 2H2O → HBr + H2SO4
- Dẫn 2 khí còn lại vào đ nước vôi trong , thấy xuất hiện vẫn đục thì đó là CO2 , không có hiện tượng gì là O2
Ca(OH)2 + CO2 → CaCO3 + H2O
a) Mg + 2HCl → MgCl2 + H2
b) nHCl = 0,05 . 3 = 0,15 mol
nMg = 1,2 : 24 = 0,05
Tỉ lệ : \(\frac{nMg}{1}< \frac{nHCl}{2}\) suy ra nHCl dư tính theo nMg
Mg + 2HCl → MgCl2 + H2
0,05mol 0,05mol 0,05 mol
=> VH2 = 0,05 . 22,4 = 1,12 lit
c) CM MgCl2= \(\frac{0,05}{0,05}=1\)M

gọi kim oxit kim loại đó là RO
n là số mol của oxit kim loại
M là nguyên tử khối của kim loại R
48 gam dd H2SO4 6,125% chứa 0,03 mol H2SO4
RO + H2SO4 ----> RSO4 + H2O
n -----> n mol
phản ứng kết thúc, H2SO4 vẫn còn dư => n < 0,03 mol
theo định luật bảo toàn khối lượng, khối lượng dung dịch sau phản ứng là:
m= n(M + 16) + 48
khối lượng H2SO4 còn lại là 98(0,03 - n)
dd T chứa H2SO4 0,98%
=> 98(0,03 - n) x 100 / [n(M + 16) + 48] = 0,98 (**)
tạm thời ta chưa biến đổi phương trình trên
dùng 2,8 lít CO để khử hoàn toàn oxit đó
RO + CO ---> R + CO2
Nhìn vào phản ứng trên ta thấy phản ứng thực chất là thay thế một phân tử CO bằng 1 phân tử CO2
=> số phân tử khí trong hỗn hợp vẫn không thay đổi
=> thể tích cũng như số mol của hỗn hợp khí sau phản ứng và trước phản ứng là giống nhau
=> sau phản ứng cũng thu được 2,8 lít hỗn hợp khí CO và CO2 (trước phản ứng chỉ có mỗi CO)
0,7 lít khí sục vào dd Ca(OH)2 dư => 0,625 gam kết tủa =>0,00625 mol CO2
0,7 lít hỗn hợp khí thì chứa 0,00625 mol CO2
=> 2,8 lít hỗn hợp khí chứa 0,025 mol CO2
theo phản ứng khử RO bằng CO thì số mol RO bằng số mol CO2
=> n = 0,025
thế n vào phương trình (**) rồi biến đổi ta tìm được M = 64
=> R là Cu
=> => a = 2 gam
sau phản ứng ta thu được 50 gam dd T gồm
0,025 mol CuSO4
0,005 mol H2SO4 còn dư
=> 20 gam dd T chứa :
0,01 mol CuSO4
0,002 mol H2SO4
phản ứng với xút (NaOH)
CuSO4 + 2NaOH ---> Cu(OH)2 + Na2SO4
0,01 --- ---> 0,02 ----- --> 0,01 ---- -->0,01 mol
H2SO4 + 2NaOH ---> Na2SO4 + H2O
0,002 -----> 0,004-----> 0,002


2SO2 + O2 \(\underrightarrow{to}\) 2SO3 (1)
SO3 + H2O → H2SO4 (2)
\(n_{SO_2}=\dfrac{8}{22,4}=\dfrac{5}{14}\left(mol\right)\)
\(m_{ddH_2SO_4.60\%}=52,7\times1,5=79,05\left(g\right)\)
\(\Rightarrow m_{H_2SO_4.60\%}=79,05\times60\%=47,43\left(g\right)\)
Theo PT1: \(n_{SO_3}=n_{SO_2}=\dfrac{5}{14}\left(mol\right)\)
\(\Rightarrow m_{SO_3}=\dfrac{5}{14}\times80=\dfrac{200}{7}\left(g\right)\)
Theo PT2: \(n_{H_2SO_4}tt=n_{SO_3}=\dfrac{5}{14}\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4}tt=\dfrac{5}{14}\times98=35\left(g\right)\)
\(m_{H_2SO_4}mới=35+47,43=82,43\left(g\right)\)
\(m_{ddH_2SO_4}mới=\dfrac{200}{7}+79,05\approx107,62\left(g\right)\)
\(\Rightarrow C\%_{ddH_2SO_4}mới=\dfrac{82,43}{107,62}\times100\%\approx76,59\%\)