Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

2K2MnO4-toK2MnO4+MnO2+O2
nKMnO4=15,8/158=0,1 mol
->nK2MnO4=nMnO2=0,05 mol
mcr=0,05.197+0,05.87=14,2g
H,=14,2/14,5=97,7%
\(n_{KMnO_4}=\dfrac{15,8}{158}=0,1mol\)
Gọi \(n_{KMnO_4}=x\)
\(2KMnO_4\rightarrow\left(t^o\right)K_2MnO_4+MnO_2+O_2\)
x 1/2 x 1/2 x ( mol )
Ta có:
\(158\left(0,1-x\right)+\dfrac{1}{2}x\left(197+87\right)=14,52\)
\(\Leftrightarrow x=0,08mol\)
\(H=\dfrac{0,08}{0,1}.100=80\%\)

$2KMnO_4\xrightarrow{t^o}K_2MnO_4+MnO_2+O_2$
$a\bigg)$
$n_{KMnO_4}=\frac{15,8}{158}=0,1(mol)$
Chất rắn sau p/ứ là $K_2MnO_4,MnO_2$
Theo PT: $n_{K_2MnO_4}=n_{MnO_2}=0,05(mol)$
$\to m_{\rm chất\, rắn}=0,05.197+0,05.87=14,2(g)$
$b\bigg)$
Vì $H=80\%\to n_{KMnO_4(p/ứ)}=0,1.80\%=0,08(mol)$
$\to n_{KMnO_4(dư)}=0,02(mol)$
Chất rắn sau p/ứ là $KMnO_4(dư):0,02;K_2MnO_4:0,04;MnO_2:0,04$
$\to m_{\rm chất\, rắn}=0,02.158+0,04.197+0,04.87=14,52(g)$
$c\bigg)$
Bảo toàn KL có:
$m_{O_2}=m_{KMnO_4}-m_{CR}$
$\to m_{O_2}=15,8-14,68=1,12(g)\to n_{O_2}=0,035(mol)$
Theo PT: $n_{KMnO_4(p/ứ)}=2n_{O_2}=0,07(mol)$
$\to H=\dfrac{0,07}{0,1}.100\%=70\%$

c2
a/ 2KMnO4(x)to→K2MnO4(0,5x)+MnO2(0,5x)+O2(0,5x)
Gọi số mol của KMnO4 tham gia phản ứng là x.
⇒mKMnO4=158x(g)
⇒mK2MnO4=0,5x.197=98,5x(g)
⇒mMnO2=0,5x.87=43,5x(g)
⇒22,12−158x+98,5x+43,5x=21,26
⇔x=0,05375(mol)
⇒VO2=0,05375.0,5.22,4=0,602(l)
b/ mKMnO4(pứ)=0,05375.158=8,4925(g)
⇒%KMnO4=8,4925\22,12.100%=38,39%

Ta có: \(n_{KMnO_4}=\dfrac{31,6}{158}=0,2\left(mol\right)\)
PT: \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
Theo PT: \(n_{K_2MnO_4}=n_{MnO_2}=n_{O_2}=\dfrac{1}{2}n_{KMnO_4}=0,1\left(mol\right)\)
\(\Rightarrow m_A=m_{K_2MnO_4}+m_{MnO_2}=0,1.197+0,1.87=28,4\left(g\right)\)
Ta có: \(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
PT: \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
Xét tỉ lệ: \(\dfrac{0,2}{3}>\dfrac{0,1}{2}\), ta được Fe dư.
Chất rắn B gồm: Fe3O4 và Fe dư.
⇒ mB = mFe3O4 + mFe (dư) = mFe + mO2 = 11,2 + 0,1.32 = 14,4 (g)

Gọi n KMnO4 = a
n KClO3 = b ( mol )
--> 158a + 122,5 b = 43,3
PTHH :
\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\uparrow\)
0,9b 1,35b
\(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
0,9a 0,45a
\(\%Mn=\dfrac{55a}{43,3-32\left(0,45a+1,35b\right)}=24,103\%\)
\(\rightarrow a=0,15\)
\(b=0,16\)
\(m_{KMnO_4}=0,15.158=23,7\left(g\right)\)
\(m_{KClO_3}=0,16.122,5=19,6\left(g\right)\)

1) \(n_{O_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
mA = mKMnO4(bđ) - mO2 = 79 - 0,15.32 = 74,2 (g)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
0,3<-----------0,15<----0,15<---0,15
=> \(H=\dfrac{0,3.158}{79}.100\%=60\%\)
2)
\(\left\{{}\begin{matrix}\%m_{K_2MnO_4}=\dfrac{0,15.197}{74,2}.100\%=39,825\%\\\%m_{MnO_2}=\dfrac{0,15.87}{74,2}.100\%=17,588\%\\\%m_{KMnO_4\left(không.pư\right)}=\dfrac{79-0,3.158}{74,2}.100\%=42,587\%\end{matrix}\right.\)
3) \(n_{KMnO_4\left(không.pư\right)}=\dfrac{79}{158}-0,3=0,2\left(mol\right)\)
PTHH: 2KMnO4 + 16HCl --> 2KCl + 2MnCl2 + 5Cl2 + 8H2O
0,2----------------------------------->0,5
K2MnO4 + 8HCl --> 2KCl + MnCl2 + 2Cl2 + 4H2O
0,15-------------------------------->0,3
MnO2 + 4Hcl --> MnCl2 + Cl2 + 2H2O
0,15------------------->0,15
=> \(V_{Cl_2}=22,4\left(0,5+0,3+0,15\right)=21,28\left(l\right)\)
\(n_{KMnO_4}=\dfrac{79}{158}=0,5mol\)
\(n_{O_2}=\dfrac{3,36}{22,4}=0,15mol\)
\(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
0,5 0,15
a)\(m_{KMnO_4}=0,15\cdot197=29,55g\)
\(m_{MnO_2}=0,15\cdot87=13,05g\)
\(m_{CRắn}=m_{KMnO_4}+m_{MnO_2}=29,55+13,05=42,6g\)
\(n_{KMnO_4pư}=0,15\cdot2=0,3mol\)
\(H=\dfrac{0,3}{0,5}\cdot100\%=60\%\)
b)\(m_{O_2}=0,15\cdot32=4,8g\)
\(\%m_{K_2MnO_4}=\dfrac{29,55}{42,6}\cdot100\%=69,37\%\)
\(\%m_{MnO_2}=100\%-69,37\%=30,63\%\)

a) $n_{O_2} = 0,15(mol)$
\(2KMnO_4\xrightarrow[]{t^o}K_2MnO_4+MnO_2+O_2\)
0,3 0,15 0,15 0,15 (mol)
$H = \dfrac{0,15.158}{63,2}.100\% = 37,5\%$
b)
$m_B = 63,2 - 0,15.32 = 58,4(gam)$
$\%m_{K_2MnO_4} = \dfrac{0,15.197}{58,4}.100\% = 50,59\%$
$\%m_{MnO_2} = \dfrac{0,15.87}{58,4}.100\% = 22,35\%$
$\%m_{KMnO_4\ dư} = 100\% -50,59\% -22,35\% = 27,06\%$
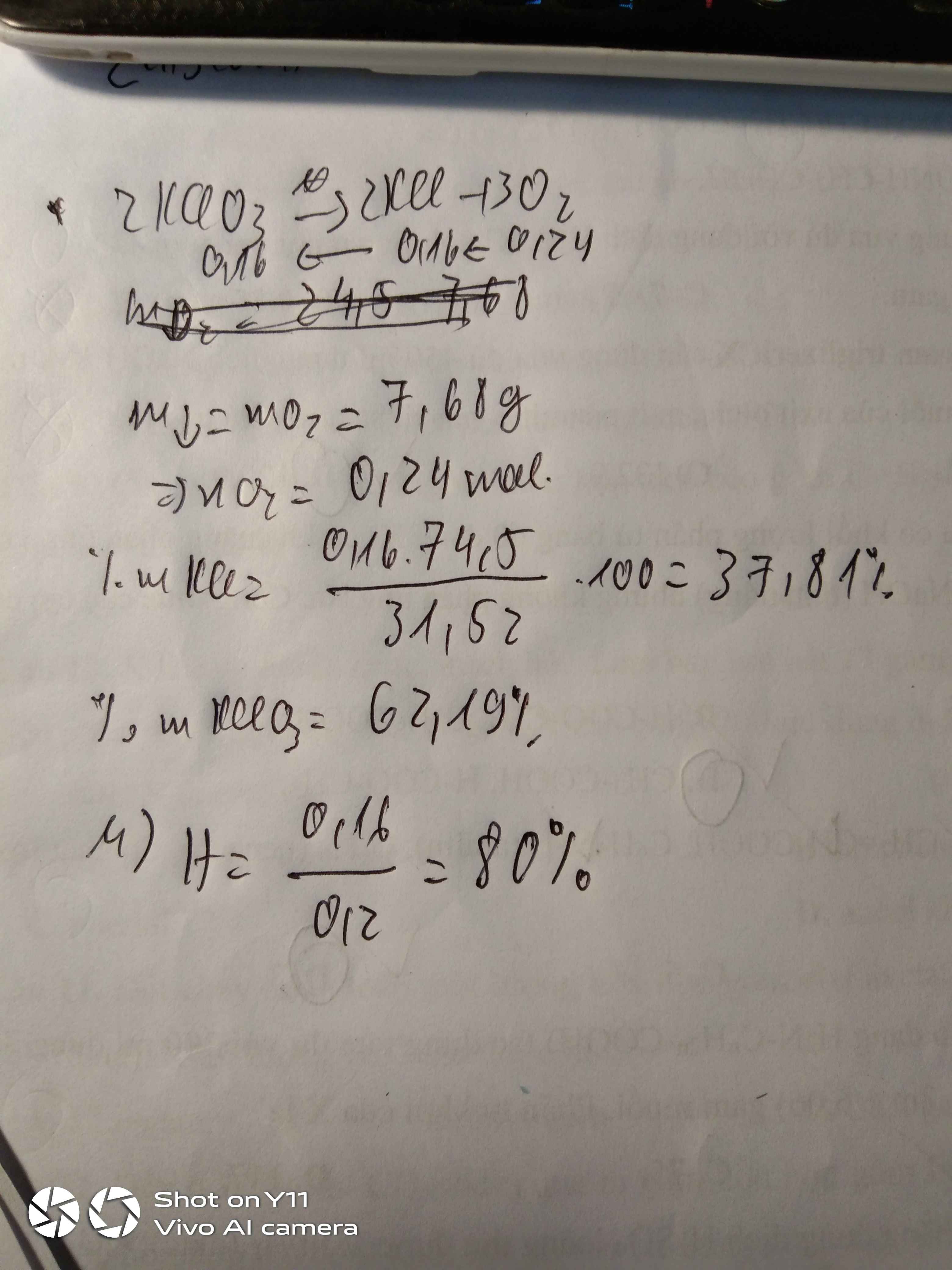
a)
PTHH :
2KMnO4 -----> K2MnO4 + MnO2 + O2
x...........................x/2...............x/2............x/2
Gọi x là số mol KMnO4 phản ứng
=> mK2MnO4 = 98,5x (g)
mMnO2 = 43,5x (g)
mKMnO4 (dư) = 31,6 - 158x (g)
=> mCR (sau) = 98,5x + 43,5x + 31,6 - 158x
=> 29,04 = -16x + 31,6
=> 2,56 = 16x
=> 0,16 = x (mol)
Theo đề bài :
mKMnO4 = 31,6 (g)
=> nKMnO4 = 31,6 : 158 = 0,2 (mol)
=> H% = 0,16 : 0,2 . 100% = 80 %
b)
Ta có :
mKMnO4 (dư) = 31,6 - 0,16 . 158 = 6,32 (g)
=> %mKMnO4 (dư) (trong X) = 6,32 : 29,04 . 100% = 21,76 (%)
mK2MnO4 = 98,5x = 15,76 (g)
=> %mK2MnO4 (trong X) = 15,76 : 29,04 . 100% = 54,27 (%)
=> %mMnO2 (trong X) = 100% - 21,76% - 54,27% = 23,97 (%)
cảm ơn