Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,PTHH:4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ b,n_{Al_2O_3}=\dfrac{30,6}{102}=0,3\left(mol\right)\\ n_{Al}=\dfrac{4}{2}.n_{Al_2O_3}=2.0,3=0,6\left(mol\right)\\ \Rightarrow m_{Al}=0,6.27=16,2\left(g\right)\\ c,n_{O_2}=\dfrac{3}{2}.n_{Al_2O_3}=\dfrac{3}{2}.0,3=0,45\left(mol\right)\\ \Rightarrow V_{O_2\left(đkc\right)}=0,45.24,79=11,1555\left(l\right)\)
a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, \(nAl_2O_3=\dfrac{30,6}{102}=0,3\left(mol\right)\)
\(nAl=\dfrac{4}{2}.0,3=0,6\left(mol\right)\)
\(mAl=0,6.27=16,2\left(g\right)\)
c, \(nO_2=\dfrac{3}{2}.0,3=0,45\left(mol\right)\)
\(VO_{2\left(đkc\right)}=0,45.24,79=11,1555\left(l\right)\)

1.\(n_{Fe_3O_4}=\dfrac{2,32}{232}=0,01mol\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
0,3 0,1 ( mol )
\(m_{Fe}=0,3.56=16,8g\)
2.\(n_{Cu}=\dfrac{3,2}{64}=0,05mol\)
\(2Cu+O_2\rightarrow\left(t^o\right)2CuO\)
0,05 0,05 ( mol )
\(m_{CuO}=0,05.80=4g\)
3.\(n_{Na}=\dfrac{4,6}{23}=0,2mol\)
\(4Na+O_2\rightarrow\left(t^o\right)2Na_2O\)
0,2 0,05 ( mol )
\(V_{O_2}=0,05.24,79=1,2395l\)
4.\(n_{Cu}=\dfrac{1,6}{64}=0,025mol\)
\(2Cu+O_2\rightarrow\left(t^o\right)2CuO\)
0,025 0,0125 ( mol )
\(V_{O_2}=0,0125.24,79=0,309875l\)

Ta có: \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
PT: \(4Al+3O_2\underrightarrow{^{t^o}}2Al_2O_3\)
Theo PT: \(n_{Al_2O_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\)
\(\Rightarrow m_{Al_2O_3}=0,1.102=10,2\left(g\right)\)
\(n_{O_2}=\dfrac{3}{4}n_{Al}=0,15\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,15.24,79=3,7185\left(l\right)\)
\(n_{Al}=\dfrac{m_{Al}}{M_{Al}}=\dfrac{5,4}{27}=0,2mol\)
PTHH: 4Al + 3O2 \(\rightarrow\) 2Al2O3
TL: 4 3 2
mol: 0,2 \(\rightarrow\) 0,15 \(\rightarrow\) 0,1
\(m_{Al_2O_3}=n_{Al_2O_3}.M_{Al_2O_3}=0,1.102=10,2g\)
\(V_{O_2}=n_{O_2}.22,4=0,15.22,4=3,36L\)

a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, \(n_{O_2}=\dfrac{7,437}{24,79}=0,3\left(mol\right)\)
Theo PT: \(n_{Al_2O_3}=\dfrac{2}{3}n_{O_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Al_2O_3}=0,2.102=20,4\left(g\right)\)
c, \(H=\dfrac{18,36}{20,4}.100\%=90\%\)

a) \(n_{SO_2}=\dfrac{6,4}{64}=0,1\left(mol\right)\)
PTHH : S + O2 - to---> SO2
0,1 0,1 0,1 ( mol )
b) \(m_S=0,1.32=3,2\left(g\right)\)
\(V_{O_2}=0,1.22,4=2,24\left(l\right)\)

\(n_{Al}=\dfrac{0,54}{27}=0,02\left(mol\right)\)
Pt : \(4Al+3O_2\xrightarrow[]{t^o}2Al_2O_3\)
0,02-->0,015-->0,01
a) \(m_{Al2O3}=0,01.102=1,02\left(g\right)\)
b) \(V_{O2\left(dktc\right)}=0,015.24,79=0,37185\left(l\right)\)
sửa lại \(V_{\left(dktc\right)}-->V_{\left(dkc\right)}\)
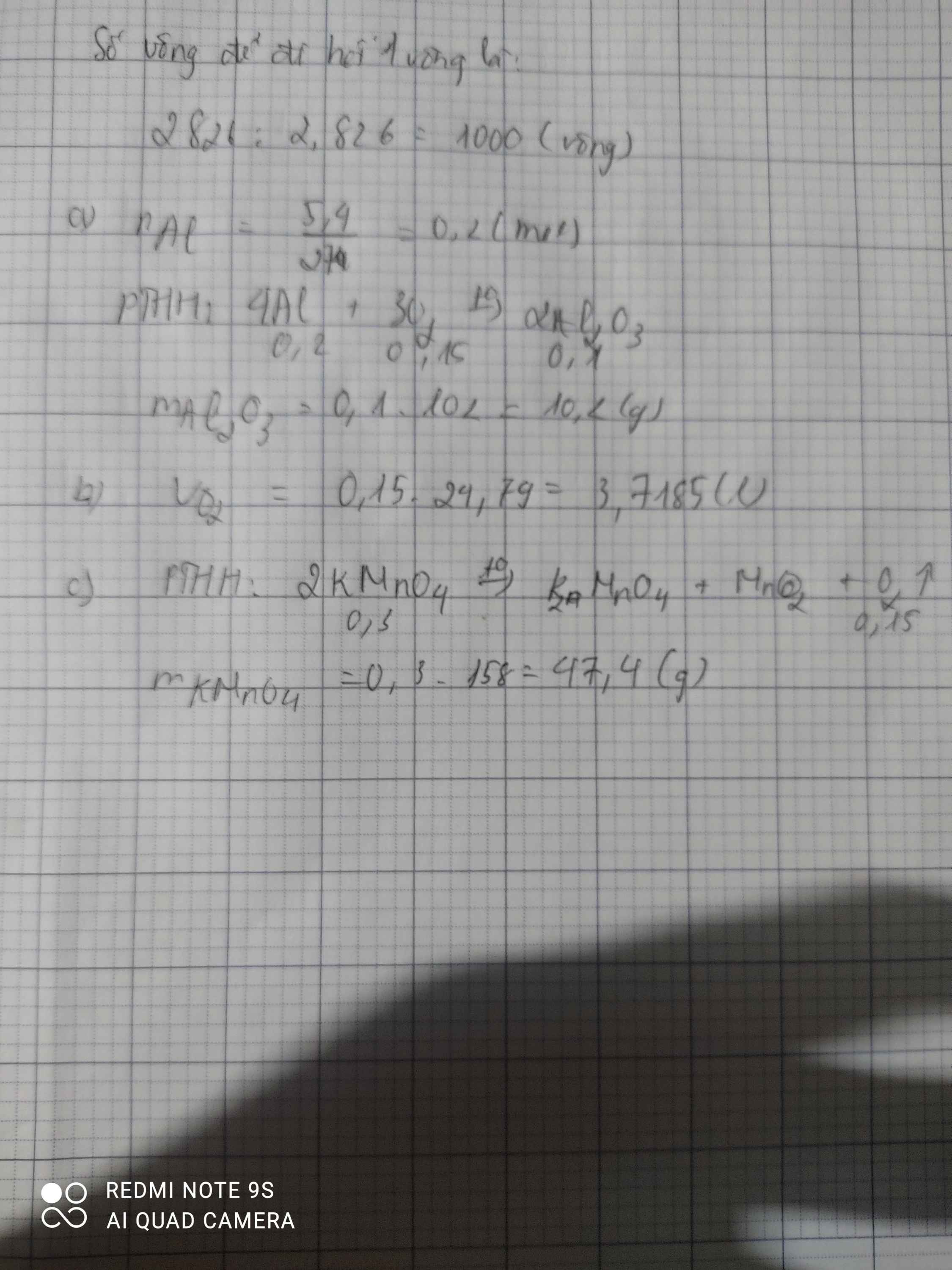
a. \(n_{KMnO_4}=\dfrac{47.4}{158}=0,3\left(mol\right)\)
PTHH : 2KMnO4 ---to----> K2MnO4 + MnO2 + O2
0,3 0,15
\(V_{O_2}=0,15.22,4=3,36\left(l\right)\)
b. PTHH : 4Al + 3O2 -> 2Al2O3
0,2 0,15
\(m_{Al}=0,2.27=5,4\left(g\right)\)
mình cảm ơn bạn ạ