Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

8.
Ta có nNa2O = 15,5:62 = 0,25 mol
a)
PTHH Na2O + H2O → 2NaOH
Phản ứng: 0,25 → 0,5 (mol)
500 ml = 0,5 lít
9.
Fe2O3 + 3H2SO4 -> Fe2(SO4)3 + 3H2O (1)
nFe2O3=0,15(mol)
Từ 1:
nFe2(SO4)3=nFe2O3=0,15(mol)
nH2SO4=3nFe2O3=0,45(mol)
mFe2(So4)3=400.0,15=60(g)
C% dd=60\124.100%=48,4%
c;
C% dd H2SO4=0,45.98\100%=44,

1. mH2SO4=98g
C%=98%-3,405%=94,595%
=>mdd sau=mH2SO4/0,94595=103,6g
=>mH2O=103,6-100=3,6
=>nH2O=0,2
=>nO trog oxit=nH2O =0,2
(giai thich: cu 1 mol H2 pu thi lay di 1 mol O trog oxit)
nFe=nH2=0,15
=>nFe:nO=0,15:0,2=3:4
=>Fe3O4.
2. nNa2SO3=0,1
=>nSO2=0,1
nFe2(SO4)3=0,3
vi la hoa 9 nen bat buoc phai viet pthh:
2FexOy+(6x-2y)H2SO4=xFe2(SO4)3+(3x-2y)S...
ti le: 0,3/x=0,1/(3x-2y)
=>x=9x-6y
=>x:y=3:4
=>Fe3O4

Câu 1:
Khối lượng CaO: 
Số mol CaO:
Pt:
 số mol Ca (OH)2
số mol Ca (OH)2
Vậy khối lượng Ca(OH)2tạo thành: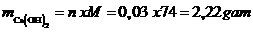
Vậy mct = 2,22 gam
 Mà
Mà 
Vậy nồng độ phần trăm Ca(OH)2: 
Câu 2:
+ Khối lượng riêng  khối lượng dd H2SO4 là
khối lượng dd H2SO4 là 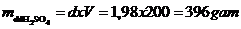
+ 
Số mol CuO: 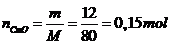
 Pt:
Pt: 

 Khối lượng
Khối lượng 
Vậy khối lượng chất tan: mct = 24 gam
Mà 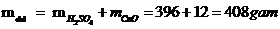
Vậy nồng độ phần trăm: 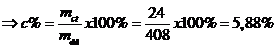

Cho một lượng dư muối Na2CO3 vào 200ml dd H2SO4 thấy thoát ra 1 chất khí, cho toàn bộ khí đó hấp thụ hoàn toàn vào 98g dd KOH 40%. Sau phản ứng làm bay hơi dung dịch thì thu được 57.6g hóa học 2 muối khan.
a, Tính khối lượng mỗi muối thu được
b. Xác định nồng độ mol của dung dịch H2SO4
___________________________________________________________________________
Na2CO3 + H2SO4 --> Na2SO4 + H2O + CO2
__a________a_________a______a_____a_
CO2 + 2KOH --> K2CO3 + H2O
_x_____2x_______x______x_
CO2 + KOH --> KHCO3
_y_____y_______y_
mKOH = 98 * 40 / 100 = 39.2 (g)
nKOH = 39.2 / 56 = 0.7 (mol)
=> 2x + y = 0.7 (1)
mmuốikhan = 138x + 100y = 57.6 (2)
giải (1) và (2) ta được
x = 0.2
y = 0.3
mà x + y = a => a = 0.5
a)
mNa2SO4 = 0.5 * 142 = 71 (g)
mK2CO3 = 0.2 * 138 = 27.6 (g)
mKHCO3 = 0.3 * 100 = 30 (g)
b)
CMddH2SO4 = 0.5 / 0.2 = 2.5 (M)
thánh chép mạng đã xuất hiện, muốn biết chi tiết câu trả lời vào: @https://diendan.hocmai.vn/threads/hoa-9-muoi-axit-bazo.274750/


Ta có nNa2SO3 = 12,6/126=0,1 (mol)
PTHH : Na2SO3 + H2SO4 \(\rightarrow\)Na2SO4 + H2O + SO2
Ta có nNa2SO3 =nSO2 = 0,1 mol
Nếu a ≤ 1 : Tạo ra muối: CaSO3↓
pt: SO2 + Ca(OH)2 --> CaSO3↓ + H2O
Ta có nSO2=nCaSO3 = 0,1 mol
=> mCaSO3= 0,1. 120=12(g)