Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(\left\{{}\begin{matrix}n_{CH_4}+n_{H_2}=\dfrac{6,72}{22,4}=0,3\\\dfrac{2.n_{H_2}}{16.n_{CH_4}+2.n_{H_2}}.100\%=30\%\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}n_{CH_4}=\dfrac{21}{310}\left(mol\right)\\n_{H_2}=\dfrac{36}{155}\left(mol\right)\end{matrix}\right.\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
\(\dfrac{21}{310}\)---------------------->\(\dfrac{21}{155}\)
2H2 + O2 --to--> 2H2O
\(\dfrac{36}{155}\)------------>\(\dfrac{36}{155}\)
=> \(m_{H_2O}=\left(\dfrac{21}{155}+\dfrac{36}{155}\right).18=\dfrac{1026}{155}\left(g\right)\)

Vì: %mCH4 = 80%
\(\Rightarrow m_{CH_4}=25.80\%=20\left(g\right)\Rightarrow n_{CH_4}=\dfrac{20}{16}=1,25\left(mol\right)\)
\(\Rightarrow m_{H_2}=5\left(g\right)\Rightarrow n_{H_2}=\dfrac{5}{2}=2,5\left(mol\right)\)
PT: \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
\(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
Theo PT: \(\Sigma n_{O_2}=\dfrac{1}{2}n_{H_2}+2n_{CH_4}=3,75\left(mol\right)\)
\(\Rightarrow V_{O_2}=3,75.22,4=84\left(l\right)\)
Mà: %VO2 = 20%
\(\Rightarrow V_{kk}=\dfrac{84}{20\%}=420\left(l\right)\)
Bạn tham khảo nhé!

a, Có: \(n_{O_2}=\dfrac{21,28}{22,4}=0,95\left(mol\right)\)
Theo ĐLBT KL, có: m + mO2 = mCO2 + mH2O
⇒ m = 28,6 + 14,4 - 0,95.32 = 12,6 (g)
b, Có: \(n_{CO_2}=\dfrac{28,6}{44}=0,65\left(mol\right)\)
\(n_{H_2O}=\dfrac{14,4}{18}=0,8\left(mol\right)\)
BTNT O, có: nCO + 2nO2 = 2nCO2 + nH2O
⇒ nCO = 0,65.2 + 0,8 - 0,95.2 = 0,2 (mol)
⇒ mCO = 0,2.28 = 5,6 (g)
\(\Rightarrow\%m_{CO}=\dfrac{5,6}{12,6}.100\%\approx44,44\%\)
Bạn tham khảo nhé!

a) Ta có: \(\overline{M}=12\cdot2=24\)
Theo phương pháp đường chéo: \(n_{CH_4}=n_{O_2}\) \(\Rightarrow\%V_{CH_4}=\%V_{O_2}=50\%\)
Giả sử \(n_{O_2}=n_{CH_4}=1\left(mol\right)\) \(\Rightarrow\left\{{}\begin{matrix}\%m_{O_2}=\dfrac{32}{32+16}\cdot100\%\approx66,67\%\\\%m_{CH_4}=33,33\%\end{matrix}\right.\)
b) Ta có: \(n_{O_2}=n_{CH_4}=\dfrac{\dfrac{16,8}{22,4}}{2}=0,375\left(mol\right)\)
PTHH: \(CH_4+3O_2\xrightarrow[]{t^o}CO_2+2H_2O\)
Theo PTHH: \(n_{CO_2}=n_{CH_4}=0,375\left(mol\right)\)
\(\Rightarrow d_{hh/CH_4}=\dfrac{44\cdot0,375+32\cdot0,375}{16}=1,78125\)

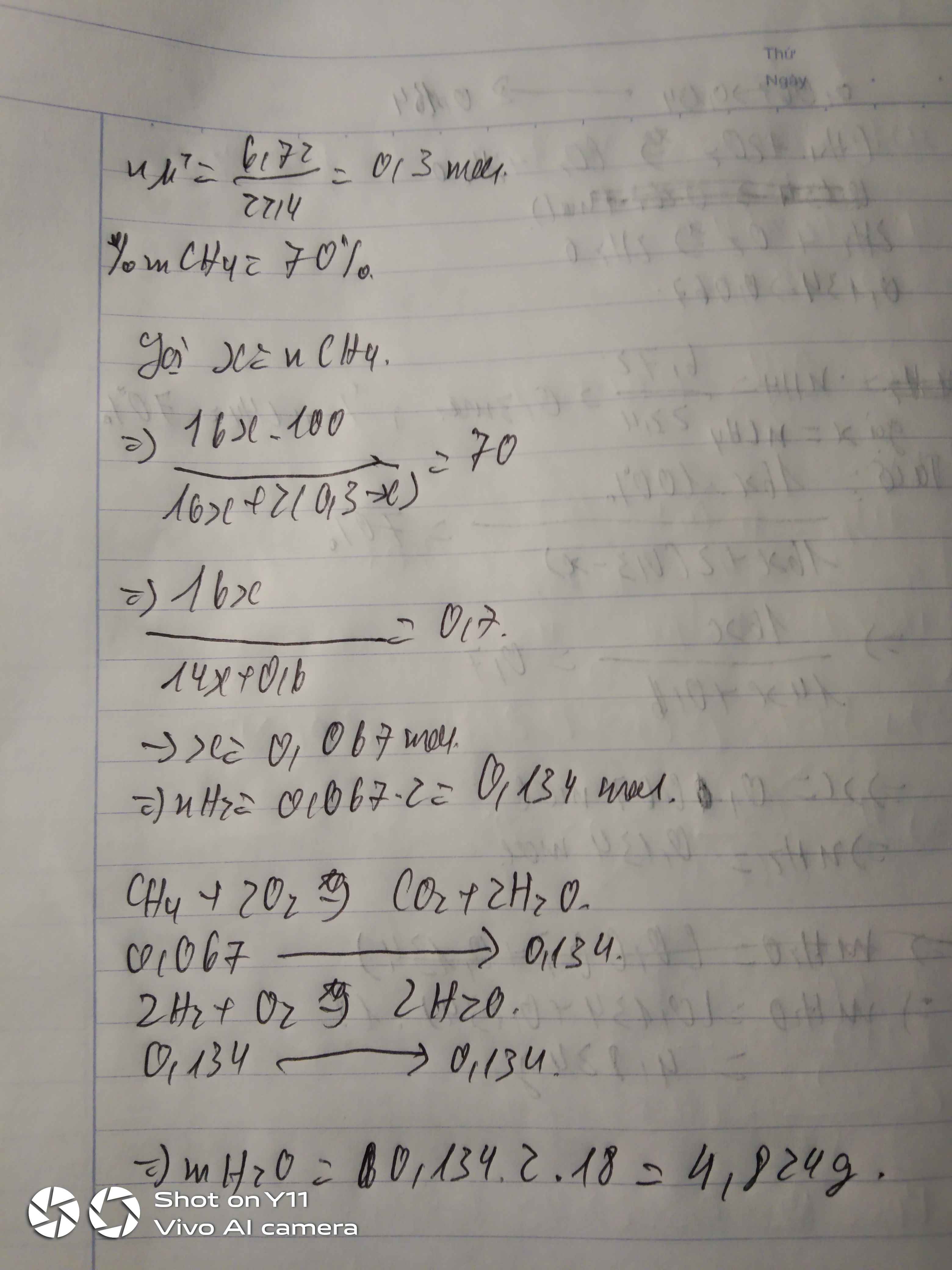
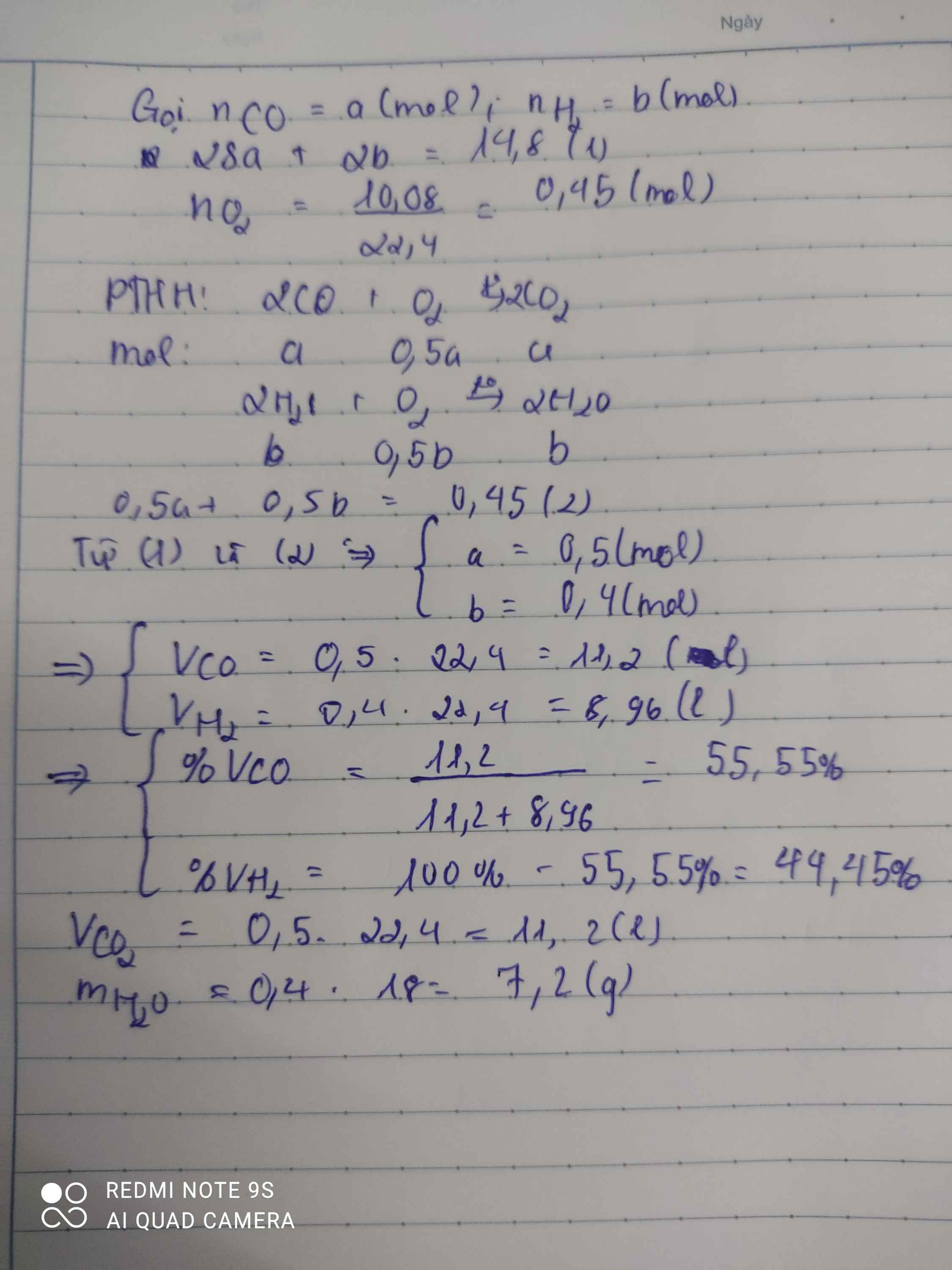
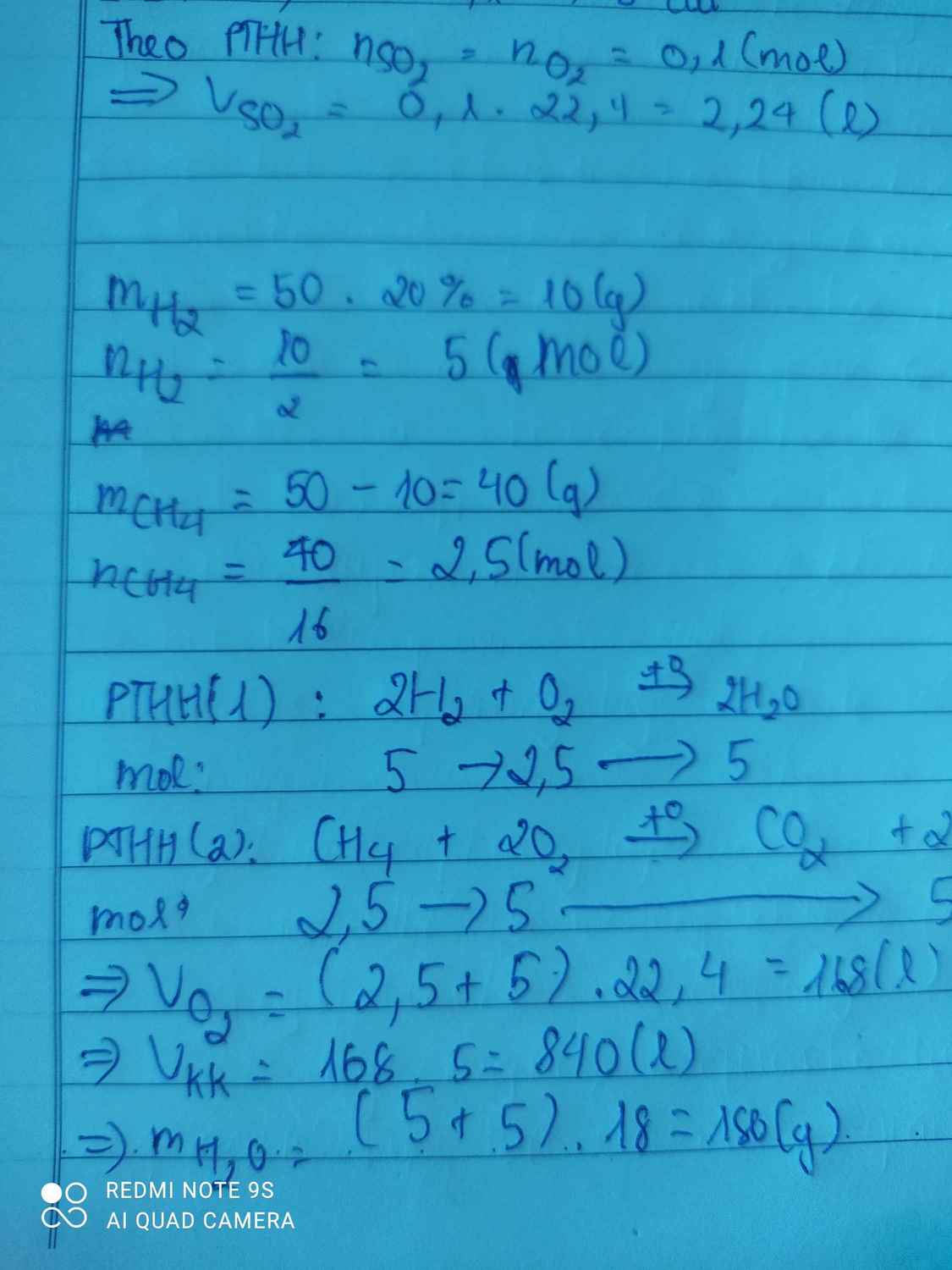
Gọi x là số mol của \(CH_4\)
\(H_2\) chiếm 20% về khối lượng -> \(CH_4\) chiếm 80%
\(n_{hh}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Ta có:\(\)\(\dfrac{16x.100\%}{16x+2\left(0,3-x\right)}=80\%\)
...........\(\dfrac{16x}{14x+0,6}=0,8\)
...........\(16x=11,2x+0,48\)
...........\(x=0,1\)=>\(n_{H_2}=0,2\)
\(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
1............2...........1.............2(mol)
0,1........0,2.........0,1........0,2(mol)
\(2H_2+O_2\underrightarrow{t^o}2H_2O\)
2...........1..........2(mol)
0,2.......0,1.......0,2(mol)
Khối lượng nước thu được
(0,2+0,2).18=7,2(g)