
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Câu 1:
\(C_2H_4+H_2O\underrightarrow{t^o,xt}C_2H_5OH\)
\(C_2H_5OH+O_2\underrightarrow{^{mengiam}}CH_3COOH+H_2O\)
\(CH_3COOH+C_2H_5OH⇌CH_3COOC_2H_5+H_2O\) (xt: H2SO4 đặc, to)
(4) \(Zn+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Zn+H_2\)
Câu 2:
a, \(n_{C_2H_4}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PT: \(C_2H_4+H_2O\underrightarrow{^{t^o,xt}}C_2H_5OH\)
Theo PT: \(n_{C_2H_5OH}=n_{C_2H_4}=0,4\left(mol\right)\Rightarrow m_{C_2H_5OH}=0,4.46=18,4\left(g\right)\)
b, \(V_{C_2H_5OH}=\dfrac{18,4}{0,8}=23\left(ml\right)\)
⇒ Độ rượu = \(\dfrac{23}{23+150}.100\approx13,3^o\)
c, \(n_{CH_3COOH}=\dfrac{120}{60}=2\left(mol\right)\)
PT: \(CH_3COOH+C_2H_5OH⇌CH_3COOC_2H_5+H_2O\) (xt: H2SO4 đặc, to)
Xé tỉ lệ: \(\dfrac{2}{1}>\dfrac{0,4}{1}\), ta được CH3COOH dư.
Theo PT: \(n_{CH_3COOC_2H_5\left(LT\right)}=n_{C_2H_5OH}=0,4\left(mol\right)\)
Mà: H = 95%
\(\Rightarrow n_{CH_3COOH\left(TT\right)}=0,4.95\%=0,38\left(mol\right)\)
\(\Rightarrow m_{CH_3COOC_2H_5\left(TT\right)}=0,38.88=33,44\left(g\right)\)


1.
\(C_{12}H_{22}O_{11}+H_2O\rightarrow C_6H_{12}O_6+C_6H_{12}O_6\)
\(C_6H_{12}O_6\xrightarrow[xt:enzim]{t^o:30-35^o}2C_2H_5OH+2CO_2\)
\(C_2H_5OH+O_2\underrightarrow{xt:men\left(giấm\right)}CH_3COOH+H_2O\)
\(CH_3COOH+C_2H_5OH\underrightarrow{H_2SO_4đặc,t^o}CH_3COOC_2H_5+H_2O\)
1.
C12H22O11+H2O→C6H12O6+C6H12O6C12H22O11+H2O→C6H12O6+C6H12O6
C6H12O6to:30−35o−−−−−→xt:enzim2C2H5OH+2CO2C6H12O6→xt:enzimto:30−35o2C2H5OH+2CO2
C2H5OH+O2xt:men(giấm)−−−−−−−−−−−−→CH3COOH+H2OC2H5OH+O2xt:men(giấm)→CH3COOH+H2O
CH3COOH+C2H5OHH2SO4đặc,to−−−−−−−−−−→CH3COOC2H5+H2O

Ví dụ : Từ câu (a) => (g)
a) \(BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl\)
\(Ba^{2+}+SO_4^{2-}\rightarrow BaSO_4\)
b) \(BaCl_2+NaOH\) ----//---->
c) \(NaCl+AgNO_3\rightarrow NaNO_3+AgCl\)
\(Ag^++Cl^-\rightarrow AgCl\)
d) \(FeCl_2+2NaOH\rightarrow Fe\left(OH\right)_2+2NaCl\)
\(Fe^{2+}+2OH^-\rightarrow Fe\left(OH\right)_2\)
e) \(Na_2S+2HCl\rightarrow2NaCl+H_2S\)
\(2H^++S^{2-}\rightarrow H_2S\)
f) \(Na_2CO_3+2HNO_3\rightarrow2NaNO_3+H_2O+CO_2\)
\(2H^++CO_3^{2-}\rightarrow CO_2+H_2O\)
g) \(CuS+HCl\)----//---->

2. a, Dẫn hỗn hợp khí qua dd Ca(OH)2 dư, CO2 bị hấp thụ tạo kết tủa trắng, sau đó lọc lấy CH4
CO2 + Ca(OH)2 -> CaCO3 + H2O
b, Dẫn qua dd Br2 dư:
- Làm Br2 mất màu -> C2H4
- Không hiện tượng -> CH4
3. a, nC2H4 = 4,48/22,4 = 0,2 (mol)
PTHH: C2H4 + Br2 -> C2H4Br2
Mol: 0,2 ---> 0,2 ---> 0,2
VddBr2 = 0,2/0,5 = 0,4 (l)
b, mC2H4Br2 = 0,2 . 188 = 37,6 (g)
4. nhh khí = 6,72/22,4 = 0,3 (mol)
Gọi nC2H4 = a (mol); nC2H2 = b (mol)
=> a + b = 0,3 (1)
28a + 26b = 8 (2)
(1)(2) => a = 0,1 (mol); b = 0,2 (mol)
%VC2H4 = 0,1/0,3 = 33,33%
%VC2H2 = 100% - 33,33% = 66,67%

\(H_2S+2KOH->K_2S+2H_2O\\ CO_2+2KOH->K_2CO_3+H_2O\\ V_{KOH\left(min\right)}=\dfrac{\dfrac{4,48}{22,4}\cdot2}{1}=0,4\left(L\right)=400\left(mL\right)\)

Câu 7:
a, \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
b, \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Theo PT: \(n_{Fe}=n_{H_2}=0,1\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,1.56}{10}.100\%=56\%\\\%m_{CuO}=44\%\end{matrix}\right.\)
c, \(n_{CuO}=\dfrac{10-0,1.56}{80}=0,055\left(mol\right)\)
Theo PT: \(n_{H_2SO_4}=n_{Fe}+n_{CuO}=0,155\left(mol\right)\)
\(\Rightarrow C\%_{H_2SO_4}=\dfrac{0,155.98}{100}.100\%=15,19\%\)
d, Theo PT: \(\left\{{}\begin{matrix}n_{FeSO_4}=n_{Fe}=0,1\left(mol\right)\\n_{CuSO_4}=n_{CuO}=0,055\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{FeSO_4}=0,1.152=15,2\left(g\right)\\m_{CuSO_4}=0,055.160=8,8\left(g\right)\end{matrix}\right.\)
Câu 8:
a, \(CuCO_3+2HCl\rightarrow CuCl_2+CO_2+H_2O\)
b, \(n_{CO_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Theo PT: \(n_{CuCO_3}=n_{CO_2}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{CuCO_3}=\dfrac{0,15.124}{20}.100\%=93\%\\\%m_{CuCl_2}=7\%\end{matrix}\right.\)
c, \(n_{HCl}=2n_{CO_2}=0,3\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,3}{0,2}=1,5\left(M\right)\)





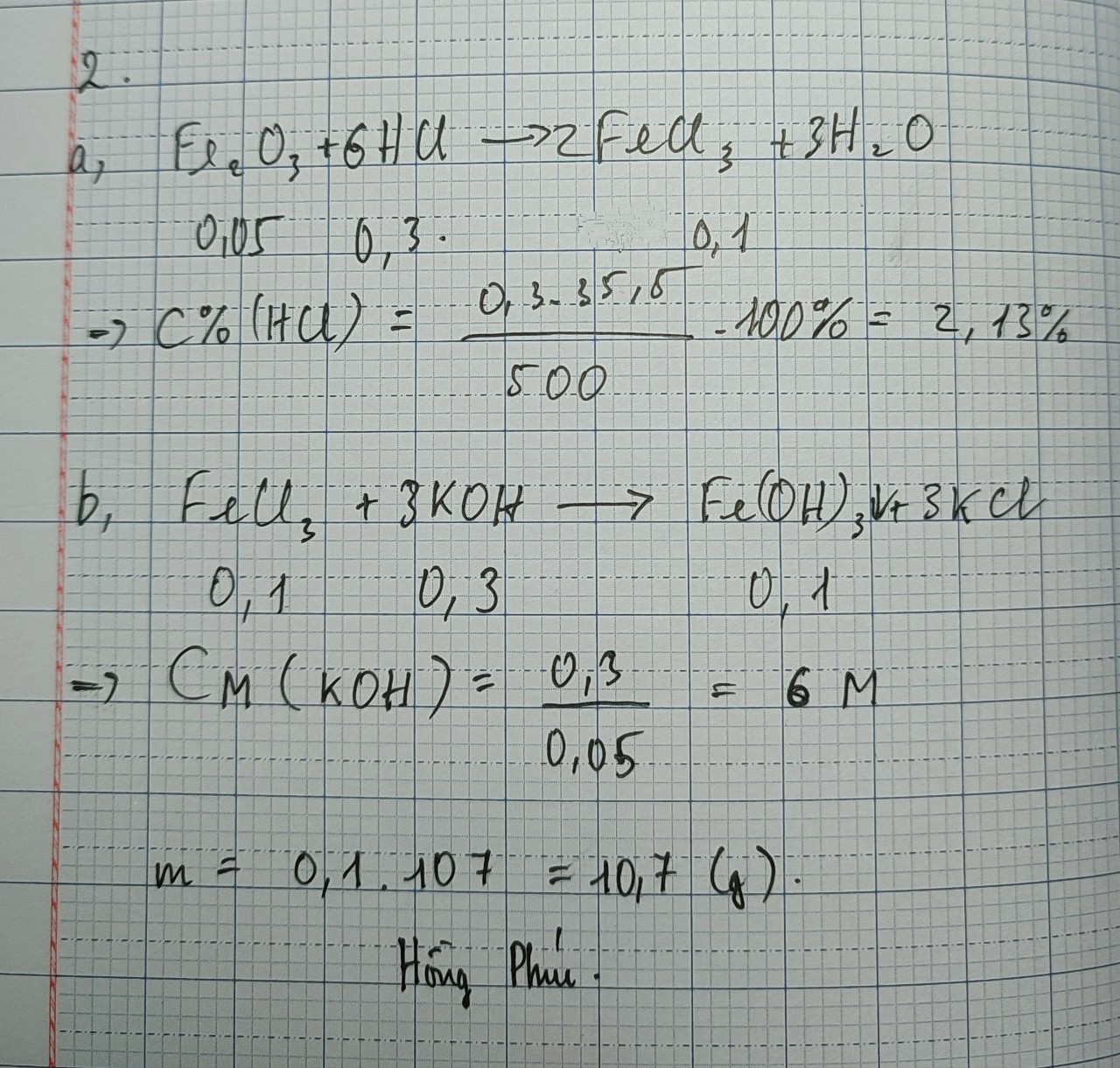


 giúp mình với ạ , mình đang cần gấp ạ, các bạn làm theo cách kẻ bảng ra giúp mình được không ạ
giúp mình với ạ , mình đang cần gấp ạ, các bạn làm theo cách kẻ bảng ra giúp mình được không ạ
\(n_{HCl}=\dfrac{3,65\%.25}{36,5}=0,025\left(mol\right)\\ Đặt:A\left(I\right)\\ 2A+2HCl\rightarrow2ACl+H_2\\ n_A=n_{HCl}=0,025\left(mol\right)\\ M_A=\dfrac{0,575}{0,025}=23\left(\dfrac{g}{mol}\right)\\ \Rightarrow A\left(I\right):Natri\left(Na=23\right)\)
=> Chọn B