Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Gọi số mol CH4, O2 là a, b (mol)
=> \(\left\{{}\begin{matrix}a+b=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\\overline{M}=\dfrac{16a+32b}{a+b}=0,4375.64=28\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}a=0,025\\b=0,075\end{matrix}\right.\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
Xét tỉ lệ: \(\dfrac{0,025}{1}< \dfrac{0,075}{2}\) => CH4 hết, O2 dư
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,025->0,05----->0,025
=> \(\left\{{}\begin{matrix}n_{CO_2}=0,025\left(mol\right)\\n_{O_2\left(dư\right)}=0,075-0,05=0,025\left(mol\right)\end{matrix}\right.\)
=> \(\%V_{CO_2}=\%V_{O_2\left(dư\right)}=\dfrac{0,025}{0,025+0,025}.100\%=50\%\)

a) \(\left\{{}\begin{matrix}n_{Cl_2}+n_{O_2}=\dfrac{6,72}{22,4}=0,3\\\overline{M}=\dfrac{71.n_{Cl_2}+32.n_{O_2}}{n_{Cl_2}+n_{O_2}}=2.29=58\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}n_{Cl_2}=0,2\left(mol\right)\\n_{O_2}=0,1\left(mol\right)\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%V_{Cl_2}=\dfrac{0,2}{0,3}.100\%=66,67\%\\\%V_{O_2}=\dfrac{0,1}{0,3}.100\%=33,33\%\end{matrix}\right.\)
b) \(\left\{{}\begin{matrix}m_{Cl_2}=0,2.71=14,2\left(g\right)\\m_{O_2}=0,1.32=3,2\left(g\right)\end{matrix}\right.\)

Giả sử các khí được đo ở điều kiện sao cho 1 mol khí chiếm thể tích 1 lít
Gọi số mol CH4, C2H6 là a, b (mol)
=> \(a+b=\dfrac{25}{1}=25\left(mol\right)\) (1)
\(n_{O_2}=\dfrac{95}{1}=95\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
a---->2a---------->a
2C2H6 + 7O2 --to--> 4CO2 + 6H2O
b------>3,5b-------->2b
=> \(\left\{{}\begin{matrix}n_{O_2\left(dư\right)}=95-2a-3,5b\left(mol\right)\\n_{CO_2}=a+2b\left(mol\right)\end{matrix}\right.\)
=> \(95-a-1,5b=\dfrac{60}{1}=60\)
=> a + 1,5b = 35 (2)
(1)(2) => a = 5; b = 20
=> \(\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{5}{25}.100\%=20\%\\\%V_{C_2H_6}=\dfrac{20}{25}.100\%=80\%\end{matrix}\right.\)
\(\overline{M}_A=\dfrac{5.16+20.30}{5+20}=27,2\left(g/mol\right)\)
\(\overline{M}_B=20,5.2=41\left(g/mol\right)\)
=> \(d_{A/B}=\dfrac{27,2}{41}\approx0,663\)

Giả sử có 1 mol khí Cl2, 2 mol khí O2
a) \(\left\{{}\begin{matrix}\%V_{Cl_2}=\dfrac{1}{1+2}.100\%=33,33\%\\\%V_{O_2}=\dfrac{2}{1+2}.100\%=66,67\%\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%m_{Cl_2}=\dfrac{1.71}{1.71+2.32}.100\%=52,59\%\\\%m_{O_2}=\dfrac{2.32}{1.71+2.32}.100\%=47,41\%\end{matrix}\right.\)
b) \(\overline{M}=\dfrac{1.71+2.32}{1+2}=45\left(g/mol\right)\)
=> \(d_{A/H_2}=\dfrac{45}{2}=22,5\)
c) \(n_A=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
=> mA = 0,3.45 = 13,5 (g)

\(n_C=\dfrac{1,2}{12}=0,1\left(mol\right)\)
PTHH: C + O2 --to--> CO2
a-->a--------->a
2C + O2 --to--> 2CO
b--->0,5b------>b
=> a + b = 0,1
Có: \(\overline{M}_X=\dfrac{44a+28b}{a+b}=16.2=32\)
=> a = 0,025; b = 0,075
\(n_{O_2}=a+0,5b=0,0625\left(mol\right)\)
=> \(V_{O_2}=0,0625.22,4=1,4\left(l\right)\)

a. 2H2+O2->2H2O
C2H2+2,5O2->2CO2+H2O
b. n hỗn hợp X=17,92/22,4=0,8
nO2=35,84/22,4=1,6
Gọi số mol H2 và C2H2 là a và b
2H2+O2->2H2O
a 0,5a
C2H2+2,5O2->2CO2+H2O
b 2,5b
Ta có a+b=0,8
Lại có 0,5a+2,5b=nO2=1,6
->a=0,2; b=0,6
->%VH2=0,2/(0,2+0,6)=25%
->%VC2H2=100%-25%=75%
%mH2=0,2.2/(0,2.2+0,6.26)=2,5%
->%mC2H2=100%-2,5%=97,5%

N phân tử = 1 mol phân tử
\(\Rightarrow n_{O2}=1mol;n_{N_2}=2mol;n_{CO_2}=1,5mol\)
\(\Rightarrow m_{hh}=1.32+2.28+1,5.44=154g\)
b. \(m_{hh}=0,1.56+0,2.64+0,3.65+0,25.27=44,65g\)
c. \(n_{O_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(n_{H_2}=\dfrac{1,12}{22,4}=0,05mol\)
\(n_{HCl}=\dfrac{6,72}{22,4}=0,3mol\)
\(n_{CO_2}=\dfrac{0,56}{22,4}=0,025mol\)
\(\Rightarrow m_{hh}=0,1.32+0,05.2+0,3.36,5+0,025.44=15,35g\)
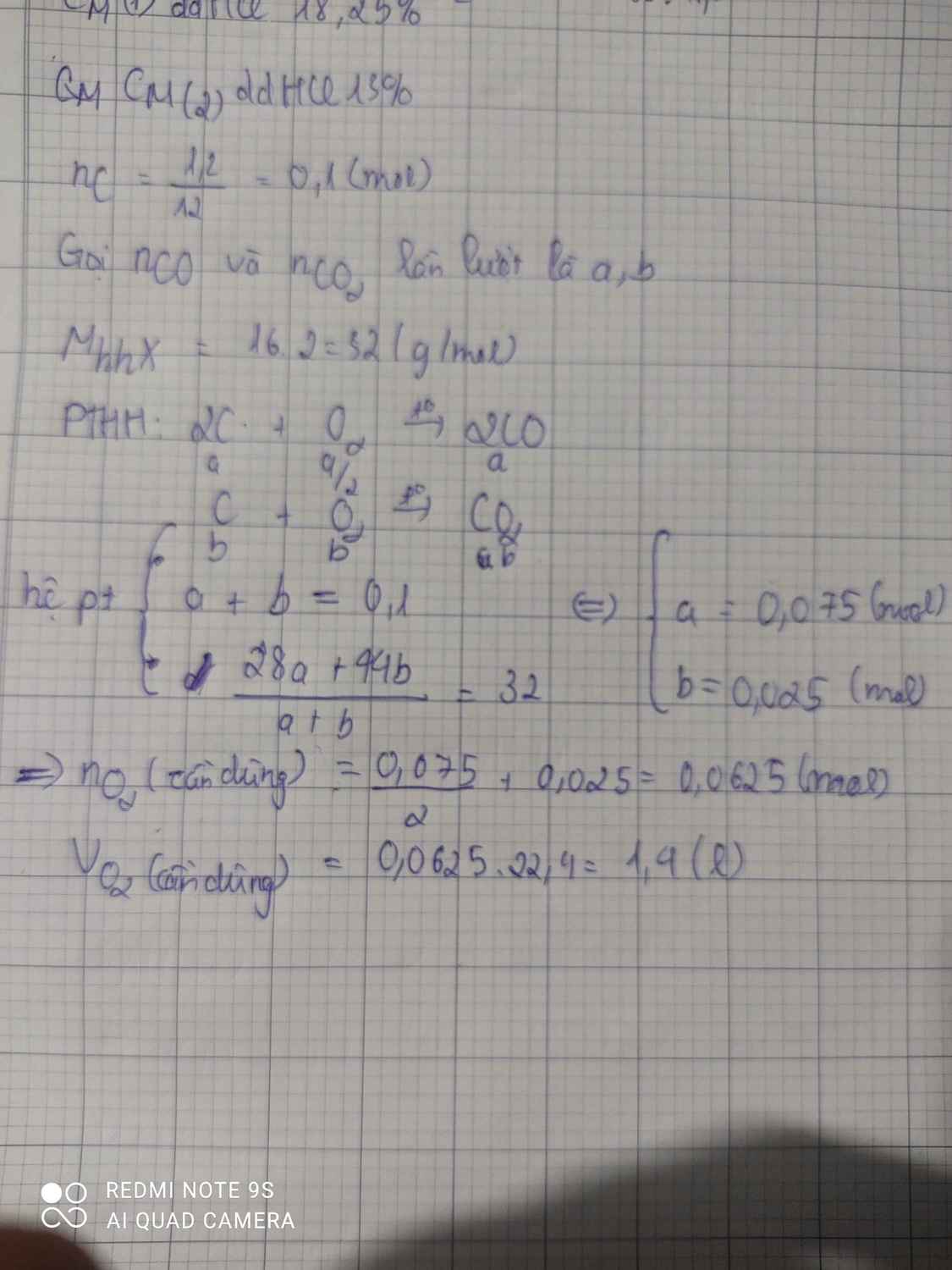
Ta có: \(n_{CO_2}=\dfrac{4,4}{44}=0,1\left(mol\right)\)
\(n_{O_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(\Rightarrow\overline{M}_B=\dfrac{m_{CO_2}+m_{O_2}+m_{H_2}}{n_{CO_2}+n_{O_2}+n_{H_2}}=\dfrac{4,4+0,1.32+0,3.2}{0,1+0,1+0,3}=16,4\left(g/mol\right)\)
\(\Rightarrow d_{B/H_2}=\dfrac{\overline{M}_B}{M_{H_2}}=\dfrac{16,4}{2}=8,2\)