Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

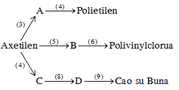
\(C_2H_4+H_2O\underrightarrow{H^+,t^o}C_2H_5OH\)
\(C_2H_5OH+O_2\underrightarrow{men.giấm}CH_3COOH+H_2O\)
\(CH_3COOH+C_2H_5OH\underrightarrow{H_2SO_{4\left(đ\right)},t^o}CH_3COOC_2H_5+H_2O\)
\(CH_3COOC_2H_5+NaOH\underrightarrow{t^o}CH_3COONa+C_2H_5OH\)
C2H4 → C2H5OH → CH3COOH → CH3COOC2H5 → C2H5OH
(1) C2H4 + H2O \(\underrightarrow{axit}\) C2H5OH
(2) C2H5OH + O2 \(\xrightarrow[25^0-30^0C]{mengiam}\) CH3COOH + H2O
(3) CH3COOH + C2H5OH → CH3COOC2H5 + H2O
(4) CH3COOC2H5 + NaOH \(\underrightarrow{t^0}\) CH3COONa + C2H5OH

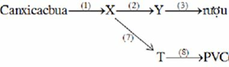
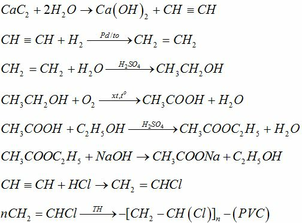
\(\left(-C_6H_{10}O_5-\right)_n+nH_2O\rightarrow nC_6H_{12}O_6\\ C_6H_{12}O_6\underrightarrow{\text{men rượu}}2C_2H_5OH+2CO_2\\ C_2H_5OH+O_2\underrightarrow{\text{men giấm}}CH_3COOH+H_2O\\ CH_3COOH+C_2H_5OH\xrightarrow[t^o]{H_2SO_{4\left(đ\right)}}CH_3COOC_2H_5+H_2O\)

a. SO 2 + Ca ( OH ) 2 → 1 : 1 CaSO 3 + H 2 O
b. Ba ( HCO 3 ) 2 + NaOH → 1 : 1 BaCO 3 + NaHCO 3 + H 2 O
c
.
2
P
+
3
Cl
2
→
2
:
3
2
PCl
3
d
.
Ca
3
(
PO
4
)
2
+
2
H
2
SO
4
→
1
:
2
2
CaSO
4
+
Ca
(
H
2
PO
4
)
2
e
.
H
3
PO
4
+
3
KOH
→
1
:
3
K
3
PO
4
+
3
H
2
O
g
.
CO
2
+
NaOH
→
1
:
1
NaHCO
3

(1) 2Na + Cl2 → 2NaCl
(2) Cl2 + H2 → 2HCl
(3) 3HCl + Fe(OH)3 → FeCl3 + 3H2O
(4) FeCl3 + Cu → CuCl2 + FeCl2

\(C_{12}H_{22}O_{11}+H_2O\underrightarrow{H^+,t^o}C_6H_{12}O_6\left(glucozo\right)+C_6H_{12}O_6\left(fructozo\right)\)
\(C_6H_{12}O_6\underrightarrow{men.rượu}2C_2H_5OH+2CO_2\)
\(C_2H_5OH+O_2\underrightarrow{men.giấm}CH_3COOH+H_2O\)
\(CH_3COOH+C_2H_5OH\underrightarrow{H_2SO_{4\left(đ\right)},t^o}CH_3COOC_2H_5+H_2O\)

Câu 1 :
$2Fe(OH)_3 \xrightarrow{t^o} Fe_2O_3 + 3H_2O$
$Fe_2O_3 + 3CO \xrightarrow{t^o} 2Fe + 3CO_2$
$Fe + 2HCl \to FeCl_2 + H_2$
$FeCl_2 + 2KOH \to Fe(OH)_2 + 2KCl$
Câu 2 :
$a) Zn + 2HCl \to ZnCl_2 + H_2$
b) Theo PTHH : $n_{H_2} = n_{Zn} = \dfrac{32,5}{65} = 0,5(mol)$
$V_{H_2} = 0,5.22,4 = 11,2(lít)$
c) $n_{ZnCl_2} = 0,5(mol) \Rightarrow m_{ZnCl_2} = 136.0,5 = 68(gam)$
d) $n_{HCl} = 2n_{Zn} = 1(mol) \Rightarrow C_{M_{HCl}} = \dfrac{1}{0,4} = 2,5M$
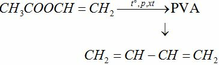
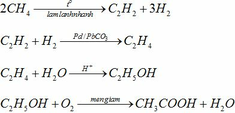
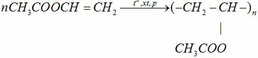
a)
\(Mg+\dfrac{1}{2}O_2\xrightarrow[]{t^o}MgO\)
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
\(MgCl_2+K_2CO_3\rightarrow2KCl+MgCO_3\downarrow\)
\(MgCO_3+2HNO_3\rightarrow Mg\left(NO_3\right)_2+H_2O+CO_2\uparrow\)
\(Mg\left(NO_3\right)_2+2KOH\rightarrow2KNO_3+Mg\left(OH\right)_2\downarrow\)
a) 2Mg + O2 ----------to---------> 2MgO
MgO + 2HCl -----------> MgCl2 + H2O
MgCl2 + K2CO3 ---------> MgCO3 + 2KCl
MgCO3 + 2HNO3 --------> Mg(NO3)2 + H2O + CO2
Mg(NO3)2 + 2KOH ----------> Mg(OH)2 + 2KNO3