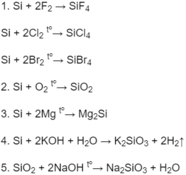
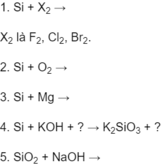Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Đáp án C
(1)NH3 + O2 ![]() NO + H2O
NO + H2O
(2)NH3 + 3CuO ![]() 3Cu + 3H2O + N2
3Cu + 3H2O + N2
(3)NH4NO3 + NaOH ![]() NaNO3 + NH3 + H2O
NaNO3 + NH3 + H2O
(4) NH4Cl ![]() NH3 + HCl
NH3 + HCl

Đáp án C
(1)NH3 + O2 → 850 ° , Pt NO + H2O
(2)NH3 + 3CuO → t ° 3Cu + 3H2O + N2
(3)NH4NO3 + NaOH → t ° NaNO3 + NH3 + H2O
(4) NH4Cl → t ° NH3 + HCl

Đáp án C
(1)NH3 + O2 → 850 ° , Pt NO + H2O
(2)NH3 + 3CuO → t ° 3Cu + 3H2O + N2
(3)NH4NO3 + NaOH → t ° NaNO3 + NH3 + H2O
(4) NH4Cl → t ° NH3 + HCl

\(CH_4 + Cl_2 \xrightarrow{as} CH_3Cl + HCl\\ CH_3-CH_3 + Cl_2 \xrightarrow{as} CH_2Cl-CH_3 + HCl\\ CH_3-CH_2-CH_3 + Cl_2 \xrightarrow{as} CH_2Cl-CH_2-CH_3 + HCl\\ CH_3-CH_2-CH_3 + Cl_2 \xrightarrow{as} CH_3-CHCl-CH_3 + HCl\\ \)
(Chỉ xét tạo sản phẩm monoclo)
\(CH≡CH + 2AgNO_3 + 2NH_3 \to Ag_2C_2 + 2NH_4NO_3\\ CH_3-C≡CH + AgNO_3 + NH_3 \to CH_3-C≡CAg + NH_4NO_3\\ CH_2=CH-C≡CH + AgNO_3 + NH_3 \to CH_2=CH-C≡CAg + NH_4NO_3\)
\(CH_4+Cl_2\underrightarrow{askt}CH_3Cl+HCl\)
\(CH_3-CH_3+Cl_2\underrightarrow{askt}CH_3-CH_2Cl+HCl\)
\(CH_3-CH_2-CH_3+Cl_2\underrightarrow{askt}CH_3-CHCl-CH_3+HCl\)
\(CH\equiv CH+2AgNO_3+2NH_3\rightarrow Ag-C\equiv C-Ag_{\downarrow}+2NH_4NO_3\)
\(CH_3-C\equiv CH+AgNO_3+NH_3\rightarrow CH_3-C\equiv C-Ag+NH_4NO_3\)
\(CH_2=CH-C\equiv CH+AgNO_3+NH_3\rightarrow CH_2=CH-C\equiv C-Ag+NH_4NO_3\)
Bạn tham khảo nhé!

\(N_2+3H_2\underrightarrow{t^o,p}2NH_3\)
\(4NH_3+5O_2\underrightarrow{t^o,xt}4NO+6H_2O\)
\(2NO+O_2\rightarrow2NO_2\)
\(4NO_2+O_2+2H_2O\rightarrow4HNO_3\)
\(Cu+4HNO_3\rightarrow Cu\left(NO_3\right)_2+2NO_2+2H_2O\)
\(2Cu\left(NO_3\right)_2\underrightarrow{t^o}2CuO+4NO_2+O_2\)

Câu 17:
\(1.\) Đặt CTHH là \(C_xH_yO_z\)
\(\%_O=100\%-40\%-6,67\%=53,33\%\\ \Rightarrow x:y:z=\dfrac{40}{12}:\dfrac{6,67}{1}:\dfrac{53,33}{16}=3,33:6,67:3,33=1:2:1\\ \Rightarrow CTDGN:\left(CH_2O\right)_n\)
\(2.\) Ta có \(M_{\left(CH_2O\right)_n}=2,143\cdot14\cdot2=60=30n\)
\(\Rightarrow n=2\)
Vậy \(CTHH_X:C_2H_4O_2\)
Câu 16:
\((1)2NH_3\xrightarrow{t^o,xt}3H_2+N_2\\ (2)N_2+O_2\buildrel{{t^o}}\over\rightleftharpoons2NO\\ (3)2NO+O_2\to 2NO_2\\ (4)4NO_2+O_2+2H_2O\to 4HNO_3\\ (5)10HNO_3+4Mg\to 4Mg(NO_3)_2+N_2O\uparrow +5H_2O\)

Al+Fe(NO3)3=Fe(NO3)3+Fe
Al(NO3)3+3NH3+3H2O=Al(OH)3+3NH4NO3
2Al(OH)3=Al2O3+3H2O (nhiệt phân)
Al2O3=2Al+3/2O2 (điện phân nóng chảy, xúc tác 3NaF.AlF3 (criolit) )
4Al+3C=Al4C3 (nung)
Al4C3+12H2O=4Al(OH)3+3CH4
CH4+2O2=CO2+2H2O
CO2+NaOH=NaHCO3
NaHCO3+NaOH=Na2CO3+H2O
Na2CO3+2HCl=2NaCl+CO2+H2O
2CO2+Ca(OH)2=Ca(HCO3)2
Ca(HCO3)2+2NaOH=Na2CO3+CaCO3+2H2O
CaCO3+2HCl=CaCl2+H2O+CO2
Al+4HNO3->Al(NO3)3+NO+2H2O
Al(NO3)3+3NaOH->Al(OH)3+3NaNO3
2Al(OH)3->Al2O3+3H2O
2Al2O3->4Al+3O2
4Al+3C->Al4C3
Al4C3+12H2O->4Al(OH)3+3CH4
CH4+2O2->CO2+2H2O
CO2+NaOH->NaHCO3
NaHCO3+NaOH->Na2CO3+H2O
Na2CO3+2HCl->2NaCl+CO2+H2O
2CO2+Ca(OH)2->Ca(HCO3)2
Ca(HCO3)2+Ca(OH)2->2CaCO3+2H2O
CaCO3+2HCl->CaCl2+CO2+H2O