Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Fe}=a\left(mol\right),n_{Al}=b\left(mol\right)\)
\(m_{hh}=56a+27b=11\left(g\right)\left(1\right)\)
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(n_{H_2}=a+1.5b=0.4\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.1,b=0.2\)
\(\%Fe=\dfrac{0.1\cdot56}{11}\cdot100\%=50.91\%\)
\(\%Al=49.09\%\)

Coi X gồm Fe và O.
Ta có: 56nFe + 16nO = 49,6 (1)
\(n_{SO_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
BT e, có: 3nFe - 2nO = 2nSO2 = 0,8 (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{Fe}=0,7\left(mol\right)\\n_O=0,65\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\%m_O=\dfrac{0,65.16}{49,6}.100\%\approx20,97\%\)
Muối thu được là Fe2(SO4)3
BTNT Fe, có: \(n_{Fe_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Fe}=0,35\left(mol\right)\)
\(\Rightarrow m_{Fe_2\left(SO_4\right)_3}=0,35.400=140\left(g\right)\)

a) nMg:a(mol) ,nAl:b(mol)
nNO=2,464/22,4=0,11(mol)
hpt: mX=24a+27b=3,42
nNO=23a+b=0,11
→a=0,075(mol),b=0,06(mol)
%mMg=(0,075.24/3,42).100%=52,63%
%mAl=100%−52,63%=47,37%
b)
nHNO3=4nNO=0,44(mol)
mdd HNO3=(0,44.63)/10%=277,2(g)

\(Đặt:\left\{{}\begin{matrix}n_{Mg}=x\left(mol\right)\\n_{Al}=y\left(mol\right)\end{matrix}\right.\\Mg+2HCl\rightarrow MgCl_2+H_2\\ 2Al+6HCl\rightarrow2AlCl_3+3H_2\\ \Rightarrow\left\{{}\begin{matrix}24x+27y=10,2\\x+1,5y=0,5\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=0,2\\y=0,2\end{matrix}\right.\\ \Rightarrow\%m_{Mg}=\dfrac{0,2.24}{10,2}.100=47,06\%\\ \%m_{Al}=52,94\%\\ n_{HCl}=2n_{Mg}+3n_{Al}=0,2.2+0,3.2=1\left(mol\right)\\ \Rightarrow V_{HCl}=\dfrac{1}{2}=0,5\left(l\right)\)

\(n_{Mg}=a\left(mol\right),n_{Al}=b\left(mol\right)\)
\(24a+27b=15\left(1\right)\)
Bảo toàn e :
\(2a+3b=0.1\cdot\left(2+3+1+8\right)\left(2\right)\)
\(\left(1\right),\left(2\right):\)
\(a=0.4,b=0.2\)
\(\%Mg=64\%,\%Al=36\%\)

\(\left\{{}\begin{matrix}n_{Cu}\\n_{Fe}\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}64x+56y=24,4\\2x+3y=\dfrac{6,72}{22,4}.3\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,285\\y=0,11\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%m_{Cu}=74,75\%\\\%m_{Fe}=25,25\%\end{matrix}\right.\)

\(c,n_{Al}=x(mol);n_{Mg}=y(mol)\\ \Rightarrow 27x+24y=10,2(1)\\ n_{H_2}=\dfrac{11,2}{22,4}=0,5(mol)\\ PTHH:2Al+6HCl\to 2AlCl_3+3H_2\\ Mg+2HCl\to MgCl_2+H_2\\ \Rightarrow 1,5x+y=0,5(2)\\ (1)(2)\Rightarrow x=y=0,2(mol)\\ \Rightarrow \%_{Al}=\dfrac{0,2.27}{10,2}.100\%=52,94\%\\ \%_{Mg}=100\%-52,94\%=47,06\%\\ d,\Sigma n_{HCl}=3x+2y=1(mol)\\ \Rightarrow V_{dd_{HCl}}=\dfrac{1}{2}=0,5(l)\)

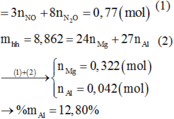
nSO2= 0,4(mol)
Đặt: nAl=a(mol); nMg=b(mol) (a,b>0)
PTHH: 2 Al + 6 H2SO4(đ) -to-> Al2(SO4)3 + 3 SO2 + 6 H2O
a____________3a______0,5a___________1,5a(mol)
Mg + 2 H2SO4(đ) -to-> MgSO4 + SO2 + 2 H2O
b_____2b________b________b(mol)
Ta có hpt:
\(\left\{{}\begin{matrix}27a+24b=7,8\\1,5a+b=0,4\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\)
=> mMg=0,1.24=2,4(g)
=>%mMg=(2,4/7,8).100=30,769%
=> %mAl= 69,231%