Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Na}=\dfrac{4,6}{23}=0,2mol\)
\(2Na+2H_2O\rightarrow2NaOH+H_2\)
0,2 0,2 0,2 0,1
\(V_{H_2}=0,1\cdot22,4=2,24l\)
\(m_{NaOH}=0,2\cdot40=8g\)
\(m_{ddNaOH}=4,6+0,2\cdot18-0,1\cdot2=8g\)
\(\Rightarrow C\%=\dfrac{m_{NaOH}}{m_{ddNaOH}}\cdot100\%=\dfrac{8}{8}\cdot100\%=100\%???\)
Sửa đề: Tính nồng độ mol của dung dịch NaOH???
\(C_{M_{NaOH}}=\dfrac{0,2}{0,3}=\dfrac{2}{3}M\)

a, \(Mg+2HCl\rightarrow MgCl_2+H_2\)
b, \(m_{Mg}=\dfrac{7,2}{24}=0,3\left(mol\right)\)
Theo PT: \(n_{MgCl_2}=n_{Mg}=0,3\left(mol\right)\Rightarrow m_{MgCl_2}=0,3.95=28,5\left(g\right)\)
c, \(n_{HCl}=2n_{Mg}=0,6\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,6}{0,2}=3\left(M\right)\)

Ba + 2H2O -- > Ba(OH)2 + H2
nBa = 27,4 / 137 = 0,2 (mol)
mBa(OH)2 = 0,2 . 171 = 34,2 (g)
VH2 = 0,2.22,4 = 4,48 (l)
VH2(thực tế ) = 4,48 .80%=3,584 (l )

\(n_{Na}=\dfrac{13,8}{23}=0,6\left(mol\right)\\ pthh:Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
0,6 0,6 0,3
\(V_{H_2}=0,3.22,4=6,72\left(l\right)\\ c,m_{\text{dd}}=13,8+286,8-\left(0,3.2\right)=300\left(g\right)\\ C\%=\dfrac{0,6.40}{300}.100\%=8\%\)
\(n_{Na}\) = \(\dfrac{13,8}{23}\) = 0,6 mol
Theo PTHH:
a) \(2Na+2H_2O\underrightarrow{t^o}2NaOH+H_2\)
2 2 2 1 (mol)
0,6 \(\rightarrow\) 0,6 \(\rightarrow\) 0,6 \(\rightarrow\) 0,3 (mol)
b) \(V_{H_2}\) = 0,3.22,4 = 6,72l
c) \(m_{dd}\) = 13,8 + 286,8 - 0,3.2 = 300g
\(C\%\) = \(\dfrac{0,6.40}{300}\).100% = 8%

a,\(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right);n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\)
PTHH: 2Na + 2H2O → 2NaOH + H2
Mol: 0,2 0,1
PTHH: 2K + 2H2O → 2KOH + H2
Mol: 0,1 0,05
b, \(n_{H_2}=0,1+0,05=0,15\left(mol\right)\)
\(V_{H_2}=0,15.22,4=3,36\left(l\right)\)
c,mdd sau pứ=4,6+3,9+91,5-0,15.2=99,7 (g)
\(\%m_{NaOH}=\dfrac{0,2.40.100\%}{99,7}=8,02\%\)
\(\%m_{KOH}=\dfrac{0,1.56.100\%}{99,7}=5,62\%\)
Bài 3 :
\(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\)
\(n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\)
a) Pt : \(2Na+2H_2O\rightarrow2NaOH+H_2|\)
2 2 2 1
0,2 0,2 0,1
\(2K+2H_2O\rightarrow2KOH+H_2|\)
2 2 2 1
0,1 0,1 0,05
b) \(n_{H2\left(tổng\right)}=0,1+0,05=0,15\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,15.22,4=3,36\left(l\right)\)
c) \(n_{NaOH}=\dfrac{0,1.2}{1}=0,2\left(mol\right)\)
⇒ \(m_{NaOH}=0,2.40=8\left(g\right)\)
\(n_{KOH}=\dfrac{0,05.2}{1}=0,1\left(mol\right)\)
⇒ \(m_{KOH}=0,1.56=5,6\left(g\right)\)
\(m_{ddspu}=8,5+91,5-\left(0,15.2\right)=99,7\left(g\right)\)
\(C_{NaOH}=\dfrac{8.100}{99,7}=8,02\)0/0
\(C_{KOH}=\dfrac{5,6.100}{99,7}=5,62\)0/0
Chúc bạn học tốt
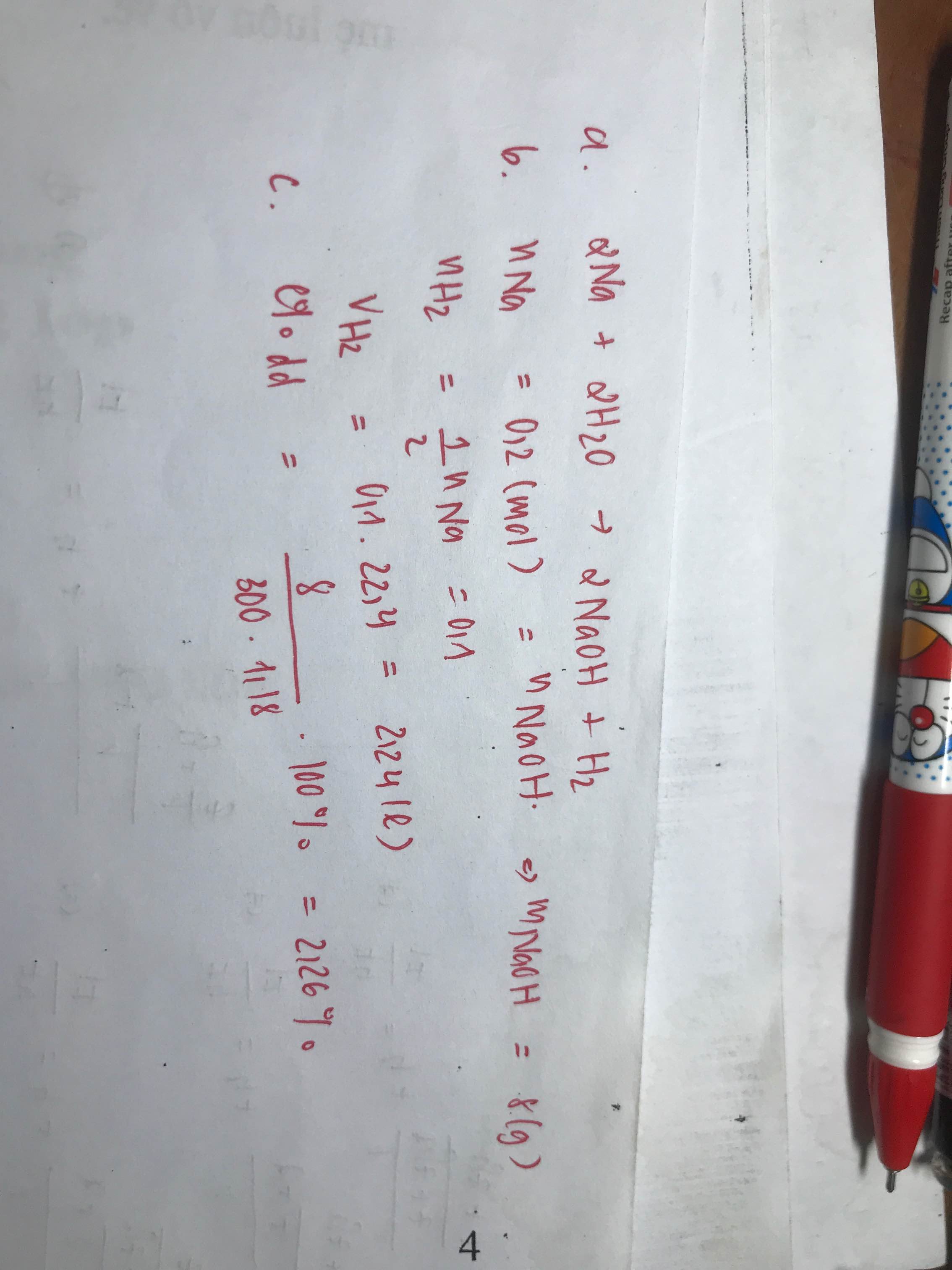
2Na + 2H2O -> 2NaOH + H2
nNa=0,2(mol)
Theo PTHH ta có:
nH2=\(\dfrac{1}{2}\)nNa=0,1(mol)
nNaOH=nNa=0,2(mol)
VH2=22,4.0,1=2,24(lít)
mNaOH=40.0,2=8(g)
C% dd NaOH=\(\dfrac{8}{300.1,18}.100\%=2,26\%\)