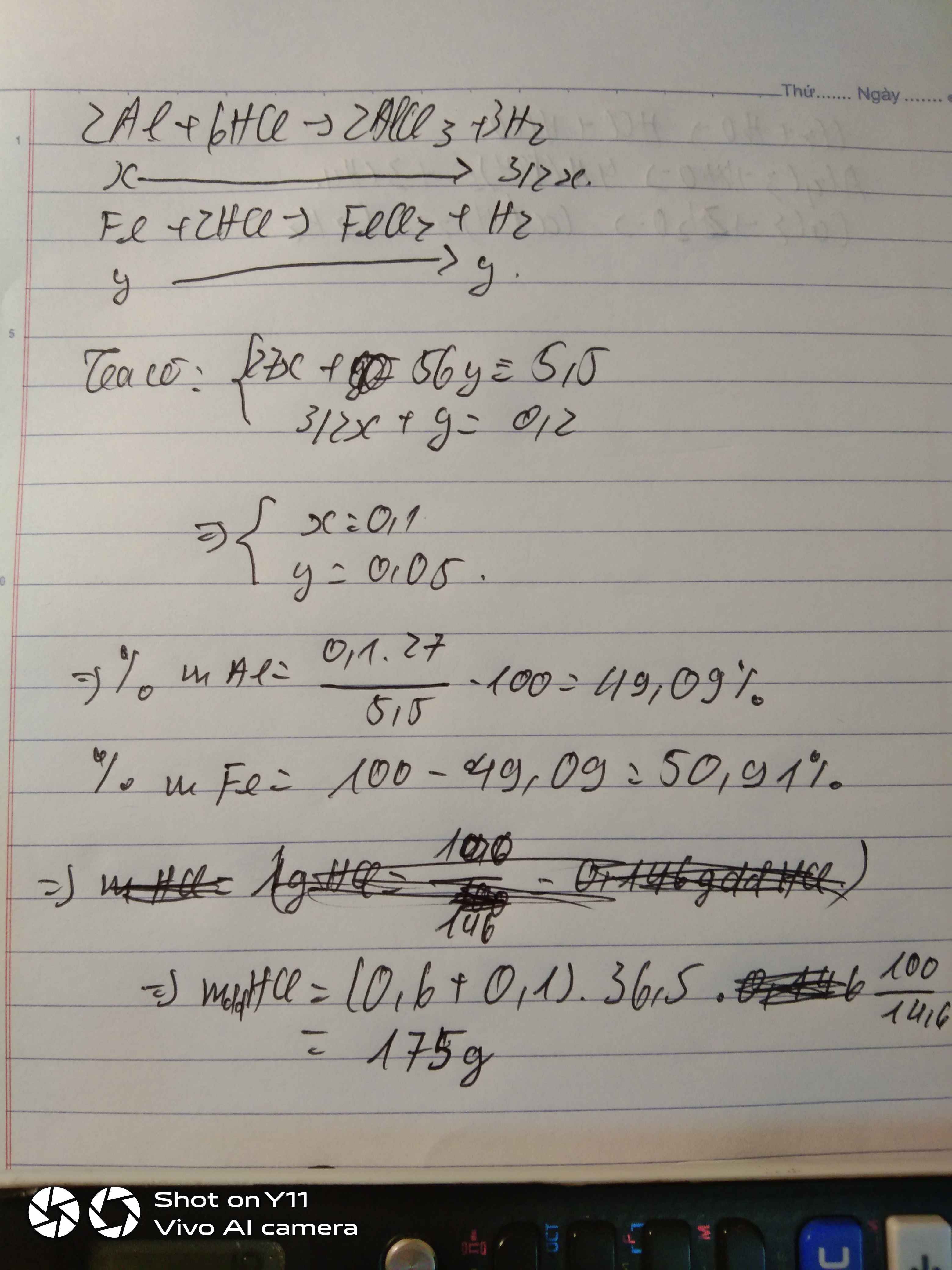Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a,Fe + 2HCl → FeCl + H2 (1)
FeO + 2HCl → FeCl + H2O (2)
nH2 = 3,36/ 22,4 = 0,15 ( mol)
Theo (1) nH2 = nFe = 0,15 ( mol)
mFe = 0,15 x 56 = 8.4 (g)
m FeO = 12 - 8,4 = 3,6 (g)
a, \(n_{H_2}=\frac{3,36}{22,4}=0,15\left(mol\right)\)
\(Fe+2HCl->FeCl_2+H_2\left(1\right)\)
\(FeO+2HCl->FeCl_2+H_2O\left(2\right)\)
theo (1) \(n_{Fe}=n_{H_2}=0,15\left(mol\right)\)
=> \(m_{Fe}=0,15.56=8,4\left(g\right)\)
=> \(m_{FeO}=12-8,4=3,6\left(g\right)\)

ta thấy : nFe =nH2 = 0,15
=> mFe =0,15 x 56 = 8,4g
%Fe=8,4/12 x 100 = 70%
=>%FeO = 100 - 70 = 30%
b) BTKLra mdd tìm mct of HCl
c) tìm mdd sau pứ -mH2 nha bạn

a) Gọi số mol Ca, CaCO3 là a, b (mol)
=> 40a + 100b = 19 (1)
\(m_{HCl}=\dfrac{500.4,38}{100}=21,9\left(g\right)\)
PTHH: Ca + 2HCl --> CaCl2 + H2
a--->2a------->a----->a
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
b------>2b------>b------>b
=> \(\overline{M}_Y=\dfrac{2a+44b}{a+b}=13,6.2=27,2\left(g/mol\right)\)
=> 25,2a = 16,8b (2)
(1)(2) => a = 0,1 (mol); b = 0,15 (mol)
\(\left\{{}\begin{matrix}m_{Ca}=0,1.40=4\left(g\right)\\m_{CaCO_3}=0,15.100=15\left(g\right)\end{matrix}\right.\)
b)
mdd sau pư = 19 + 500 - 0,1.2 - 0,15.44 = 512,2 (g)
mHCl(dư) = 21,9 - 36,5(2a + 2b) = 3,65 (g)
mCaCl2 = 111(a + b) = 27,75 (g)
\(\left\{{}\begin{matrix}C\%_{CaCl_2}=\dfrac{27,75}{512,2}.100\%=5,418\%\\C\%_{HCl\left(dư\right)}=\dfrac{3,65}{512,2}.100\%=0,713\%\end{matrix}\right.\)

a) Gọi số mol Ca, CaCO3 là a, b (mol)
=> 40a + 100b = 19 (1)
\(m_{HCl}=\dfrac{500.4,38}{100}=21,9\left(g\right)\)
PTHH: Ca + 2HCl --> CaCl2 + H2
a--->2a------->a----->a
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
b------>2b------>b------>b
=> \(\overline{M}_Y=\dfrac{2a+44b}{a+b}=13,6.2=27,2\left(g/mol\right)\)
=> 25,2a = 16,8b (2)
(1)(2) => a = 0,1 (mol); b = 0,15 (mol)
\(\left\{{}\begin{matrix}m_{Ca}=0,1.40=4\left(g\right)\\m_{CaCO_3}=0,15.100=15\left(g\right)\end{matrix}\right.\)
b)
mdd sau pư = 19 + 500 - 0,1.2 - 0,15.44 = 512,2 (g)
mHCl(dư) = 21,9 - 36,5(2a + 2b) = 3,65 (g)
mCaCl2 = 111(a + b) = 27,75 (g)
\(\left\{{}\begin{matrix}C\%_{CaCl_2}=\dfrac{27,75}{512,2}.100\%=5,418\%\\C\%_{HCl\left(dư\right)}=\dfrac{3,65}{512,2}.100\%=0,713\%\end{matrix}\right.\)

Gọi \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{4,48}{22,4}=0,2mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
Theo pt: \(\Rightarrow\left\{{}\begin{matrix}3x+y=0,2\\27x+56y=5,5\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=\dfrac{19}{470}\\y=\dfrac{37}{470}\end{matrix}\right.\)
\(\%m_{Al}=\dfrac{\dfrac{19}{470}\cdot27}{5,5}\cdot100\%=19,84\%\)
\(\%m_{Fe}=100\%-19,84\%=80,16\%\)

PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
a) Ta có: \(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)=n_{Mg}\)
\(\Rightarrow\%m_{Mg}=\dfrac{0,5\cdot24}{16}=75\%\) \(\Rightarrow\%m_{MgO}=25\%\)
b) Ta có: \(\left\{{}\begin{matrix}n_{Mg}=0,5\left(mol\right)\\n_{MgO}=\dfrac{16\cdot25\%}{40}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{HCl}=2n_{Mg}+2n_{MgO}=1,2\left(mol\right)\) \(\Rightarrow m_{ddHCl}=\dfrac{1,2\cdot36,5}{20\%}=219\left(g\right)\)
c) Theo các PTHH: \(\left\{{}\begin{matrix}n_{H_2}=0,5\left(mol\right)\\n_{MgCl_2}=0,6\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{H_2}=0,5\cdot2=1\left(g\right)\\m_{MgCl_2}=0,6\cdot95=57\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{hhA}+m_{ddHCl}-m_{H_2}=234\left(g\right)\) \(\Rightarrow C\%_{MgCl_2}=\dfrac{57}{234}\cdot100\%\approx24,36\%\)
Cho mình hỏi ở cái PTHH ấy! sao ta không tính số mol ở dưới??