Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(Đặt:\)
\(\left\{{}\begin{matrix}n_{Mg}=x\left(mol\right)\\\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{6.72}{22.4}=0.3\left(mol\right)\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\\ Fe+2HCl\rightarrow FeCl_2+H_2\)
\(m_{hh}=24x+56y=13.6\left(g\right)\\ n_{H_2}=x+y=0.3\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}x=0.1\\y=0.2\end{matrix}\right.\)
\(\%Mg=\dfrac{0.1\cdot24}{13.6}\cdot100\%=17.64\%\\ \%Fe=100-17.64=82.36\%\)
\(n_{HCl}=2n_{H_2}=2\cdot0.3=0.6\left(mol\right)\)
\(V_{HCl}=\dfrac{0.6}{2}=0.3\left(l\right)\)
\(m_Y=m_{MgCl_2}+m_{FeCl_2}=0.1\cdot95+0.2\cdot127=34.9\left(g\right)\)

1)
\(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\Rightarrow n_{HCl}=0,25.2=0,5\left(mol\right)\)
\(V=\dfrac{0,5}{0,5}=1\left(l\right)\)
2)
\(n_{NaCl}=\dfrac{5,85}{58,5}=0,1\left(mol\right)\); \(n_{AgNO_3}=\dfrac{34}{170}=0,2\left(mol\right)\)
PTHH: NaCl + AgNO3 --> NaNO3 + AgCl
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,2}{1}\) => NaCl hết, AgNO3 dư
PTHH: NaCl + AgNO3 --> NaNO3 + AgCl
0,1------------------------>0,1
=> mAgCl = 0,1.143,5 = 14,35 (g)

\(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\)
Pt : \(Fe+H_2SO_4\rightarrow FeSO_4+H_2|\)
1 1 1 1
0,15 0,15 0,15
\(n_{H2}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,15.22,4=3,36\left(l\right)\)
\(n_{H2SO4}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
300ml = 0,3l
\(C_{MddH2SO4}=\dfrac{0,15}{0,3}=0,5\left(M\right)\)
Chúc bạn học tốt

a) \(Na_2O+H_2O\rightarrow2NaOH\)
\(n_{NaOH}=2n_{Na_2O}=2.\dfrac{11,16}{62}=0,32\left(mol\right)\)
\(C\%_{NaOH}=\dfrac{0,32.40}{11,16+88,84}.100=12,8\%\)
b) \(n_{Fe}=\dfrac{4,48}{56}=0,08\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(n_{H_2}=n_{Fe}=0,08\left(mol\right)\\ \Rightarrow V_{H_2}=0,08.22,4=1,792\left(lít\right)\)
\(n_{HCl}=2n_{Fe}=0,16\left(mol\right)\)
\(m_{ddHCl}=\dfrac{0,16.36,5}{7,3\%}=80\left(g\right)\)
\(n_{FeCl_2}=n_{Fe}=0,08\left(mol\right)\\ m_{ddsaupu}=4,48+80-0,08.2=84,32\left(g\right)\)
\(C\%_{FeCl_2}=\dfrac{0,08.127}{84,32}.100=12,05\%\)

\(a)n_{Mg} = a ; n_{Al} = b \Rightarrow 24a +27b = 5,1(1)\\ Mg + 2HCl \to MgCl_2 + H_2\\ 2Al + 6HCl \to 2AlCl_3 + 3H_2\\ n_{H_2} = a + 1,5b = \dfrac{5,6}{22,4} = 0,25(2)\\ (1)(2) \Rightarrow a = 0,1 ; b = 0,1\\ \%m_{Mg} = \dfrac{0,1.24}{5,1}.100\% = 44,44\%\ ;\ \%m_{Al} = 100\% -44,44\% = 55,56\%\\ b) n_{MgCl_2} = n_{Mg} = 0,1 \Rightarrow m_{MgCl_2} = 0,1.95 = 9,5(gam)\\ n_{AlCl_3} = n_{Al} = 0,1 \Rightarrow m_{AlCl_3} = 0,1.133,5 = 13,35(gam)\\ c)n_{HCl} = 2n_{Mg} + 3n_{Al} = 0,5(mol) \Rightarrow m_{dd\ HCl} = \dfrac{0,5.36,5}{3,65\%} = 500(gam)\)
\(m_{dd\ sau\ pư} = 5,1 + 500 - 0,25.2 = 504,6(gam)\\ C\%_{MgCl_2} = \dfrac{9,5}{504,6}.100\% = 1,89\%\\ C\%_{AlCl_3} = \dfrac{13,35}{504,6}.100\% = 2,65\%\)

a: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,2 0,6 0,2 0,3
\(V=0.3\cdot22.4=6.72\left(lít\right)\)
b: \(C_{M\left(HCL\right)}=\dfrac{0.6}{0.2}=3\left(M\right)\)



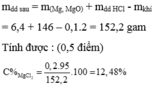
nMg = 3.6/24 = 0.15 (mol)
nAgNO3 = 0.2*0.1 = 0.02 (mol)
Mg + 2AgNO3 => Mg(NO3)2 + 2Ag
0.01........0.02
=> nMg ( Pư với HBr) = 0.15 - 0.01 = 0.14 (mol)
Mg + 2HBr => MgBr2 + H2
0.14___0.28_________0.14
CM HBr = 0.28/0.3 = 0.93 (M)
VH2 = 0.14*22.4 = 3.136 (l)