Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a. \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
\(n_{Fe}=\frac{11,2}{56}=0,2mol\)
b. Theo phương trình \(n_{HCl}=n_{Fe}.2=0,2.2=0,4mol\)
\(\rightarrow V_{ddHCl}=\frac{0,4}{2}=0,2l=200ml\)
c. Theo phương trình \(n_{FeCl_2}=n_{Fe}=0,2mol\)
\(\rightarrow C_{M_{ddFeCl_2}}=\frac{0,2}{0,2}=1M\)

a) \(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
PTHH: 2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
0,4-->0,6---------->0,2------->0,6
=> \(C_{M\left(dd.H_2SO_4\right)}=\dfrac{0,6}{0,15}=4M\)
b) VH2 = 0,6.22,4 = 13,44 (l)
c) \(C_{M\left(Al_2\left(SO_4\right)_3\right)}=\dfrac{0,2}{0,15}=\dfrac{4}{3}M\)
\(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\\
pthh:2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,4 0,6 0,2 0,6
\(C_M_{H_2SO_4}=\dfrac{0,6}{0,15}=4M\\ V_{H_2}=0,622,4=13,44L\)
\(C_M=\dfrac{0,2}{0,15}=1,3M\)

\(a.Fe+2HCl\rightarrow FeCl_2+H_2\\ b.n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\\ TheoPT:n_{HCl}=2n_{Fe}=0,2\left(mol\right)\\ \Rightarrow CM_{HCl}=\dfrac{0,2}{0,2}=1M\)

a) \(n_{Fe}=\dfrac{2,8}{56}=0,05\left(mol\right)\)
PTHH: `Fe + 2HCl -> FeCl_2 + H_2`
0,05->0,1----->0,05---->0,05
`=> V_{ddHCl} = (0,1)/2 = 0,05 (l)`
b) `V_{H_2} = 0,05.22,4 = 1,12 (l)`
c) `C_{M(FeCl_2)} = (0,05)/(0,05) = 1M`

\(n_{NaOH}=0.2\cdot0.5=0.1\left(mol\right)\)
\(n_{CuSO_4}=0.1\cdot2=0.2\left(mol\right)\)
\(2NaOH+CuSO_4\rightarrow Na_2SO_4+Cu\left(OH\right)_2\)
\(0.1.............0.05...............0.05...........0.05\)
\(m_{Cu\left(OH\right)_2}=0.05\cdot98=4.9\left(g\right)\)
\(C_{M_{Na_2SO_4}}=\dfrac{0.05}{0.2+0.1}=0.167\left(M\right)\)
\(C_{M_{CuSO_4\left(dư\right)}}=\dfrac{0.2-0.05}{0.1}=1.5\left(M\right)\)

\(a)Mg+2HCl\rightarrow MgCl_2+H_2\)
\(b)n_{Mg}=\dfrac{3}{24}=0,125mol\\ n_{HCl}=0,1.1=0,1mol\\ \Rightarrow\dfrac{0,125}{1}>\dfrac{0,1}{2}\Rightarrow Mg.dư\\ n_{H_2}=n_{MgCl_2}=\dfrac{0,1}{2}=0,05mol\\ V_{H_2}=0,05.24,79=1,2395l\\ c)C_{M_{MgCl_2}}=\dfrac{0,05}{0,1}=0,5M\)
Đoạn xét tỉ lệ phải là \(\dfrac{0,125}{1}>\dfrac{0,1}{2}\) em nhé.
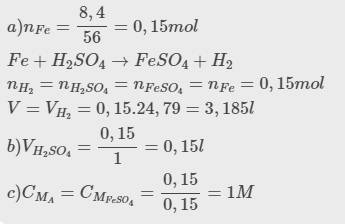
\(n_{Fe}=\dfrac{2,8}{56}=0,05\left(mol\right)\\ a,PTHH:Fe+2HCl\rightarrow FeCl_2+H_2\\ n_{H_2}=n_{FeCl_2}=n_{Fe}=0,05\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\\ V_{H_2\left(đkc\right)}=0,05.24,79=1,2395\left(l\right)\\ b,n_{HCl}=0,1.3=0,3\left(mol\right)\\ Vì:\dfrac{0,05}{1}< \dfrac{0,3}{2}\Rightarrow HCldư\\ n_{HCl\left(dư\right)}=0,3-0,05.2=0,2\left(mol\right)\\ n_{FeCl_2}=n_{Fe}=0,05\left(mol\right)\\ V_{ddsau}=V_{ddHCl}=0,1\left(l\right)\\ b,C_{MddFeCl_2}=\dfrac{0,05}{0,1}=0,5\left(M\right);C_{MddHCl\left(dư\right)}=\dfrac{0,2}{0,1}=2\left(M\right)\)