
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


nC = 11,2/22,4 = 0,5 (mol)
nH = 2 . 13,5/18 = 1,5 (mol)
nO = (11,5 - 0,5 . 12 - 1,5)/16 = 0,25 (mol)
M(A) = 32 . 1,4375 = 46 (g/mol)
CTPT: CxHyOz
=> x : y : z = 0,5 : 1,5 : 0,25 = 2 : 6 : 1
=> (C2H6O)n = 46
=> n = 1
CTPT: C2H6O
CTCT: CH3-CH2-OH hoặc CH3-O-CH3


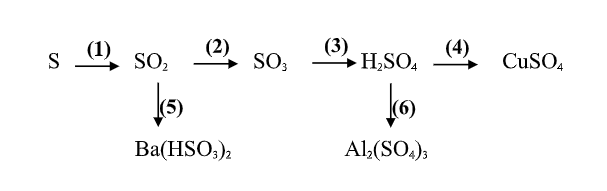
a, \(Na_2SO_3+H_2SO_4\rightarrow Na_2SO_4+SO_2+H_2O\)
\(K_2SO_3+H_2SO_4\rightarrow K_2SO_4+SO_2+H_2O\)
\(n_{SO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo PT: \(n_{H_2SO_4}=n_{SO_2}=0,3\left(mol\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{0,3.98}{20\%}=147\left(g\right)\)
b, Có: 126nNa2SO3 + 158nK2SO3 = 44,2 (1)
Theo PT: \(n_{Na_2SO_3}+n_{K_2SO_3}=n_{SO_2}=0,3\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{Na_2SO_3}=0,1\left(mol\right)\\n_{K_2SO_3}=0,2\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}n_{Na_2SO_4}=n_{Na_2SO_3}=0,1\left(mol\right)\\n_{K_2SO_4}=n_{K_2SO_3}=0,2\left(mol\right)\end{matrix}\right.\)
Ta có: m dd sau pư = 44,2 + 147 - 0,3.64 = 172 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{Na_2SO_4}=\dfrac{0,1.142}{172}.100\%\approx8,26\%\\C\%_{K_2SO_4}=\dfrac{0,2.174}{172}.100\%\approx20,23\%\end{matrix}\right.\)
c, \(n_{Ba\left(OH\right)_2}=0,5.1=0,5\left(mol\right)\)
Có: \(\dfrac{n_{SO_2}}{n_{Ba\left(OH\right)_2}}=0,6< 1\) → pư tạo muối trung hòa, Ba(OH)2 dư.
PT: \(SO_2+Ba\left(OH\right)_2\rightarrow BaSO_3+H_2O\)
Theo PT: \(n_{BaSO_3}=n_{SO_2}=0,3\left(mol\right)\Rightarrow m_{BaSO_3}=0,3.217=65,1\left(g\right)\)
\(n_{SO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH:
\(Na_2SO_3+H_2SO_4\rightarrow Na_2SO_4+H_2O+SO_2\)
x x x x
\(K_2SO_3+H_2SO_4\rightarrow K_2SO_4+H_2O+SO_2\)
y y y y y
\(\left\{{}\begin{matrix}126x+158y=44,2\\x+y=0,3\end{matrix}\right.\)
\(\Leftrightarrow x=0,1;y=0,2\)
\(a,m_{H_2SO_4}=0,3.98=29,4\left(g\right)\)
\(m_{ddH_2SO_4}=\dfrac{29,4.100}{20}=147\left(g\right)\)
\(b,C\%_{Na_2SO_4}=\dfrac{0,1.142}{44,2+147-\left(0,3.44\right)}=8,26\left(\%\right)\)
\(C\%_{K_2SO_4}=\dfrac{0,2.174}{44,2+147-\left(0,3.44\right)}=20,23\left(\%\right)\)
\(c,SO_2+Ba\left(OH\right)_2\rightarrow BaSO_3+H_2O\)
0,3 0,3 0,3
\(\dfrac{0,3}{1}< \dfrac{0,5}{1}\) --> Ba(OH)2 dư
\(m_{BaSO_3}=217.0,3=65,1\left(g\right)\)

\(n_{H_2O}=\dfrac{9}{18}=0,5\left(mol\right)\)
Bảo toàn H: nH(A) = 1 (mol)
=> mC = 7 - 1 = 6(g)
=> \(n_C=\dfrac{6}{12}=0,5\left(mol\right)\)
=> nC : nH = 0,5 : 1 = 1:2
=> CTPT: (CH2)n
Mà MA = 32.2,1875 = 70 (g/mol)
=> n = 5
=> CTPT: C5H10
CTCT:
(1) CH2 = CH – CH2 – CH2 – CH3
(2) CH3 – CH = CH – CH2 – CH3
(3)
(4)
(5)

Gọi CTHH : CxHy
\(n_{CO_2}=0,3\left(mol\right);n_{H_2O}=0.4\left(mol\right)\)
PT : 4CxHy + (4x + y)O2 \(\rightarrow\) 4xCO2 + yH2O
\(\Rightarrow\dfrac{4x}{2y}=\dfrac{0,3}{0,4}\Leftrightarrow\dfrac{x}{y}=\dfrac{3}{8}\Rightarrow x=3;y=8\) =
CTHH C3H8
b)Công thức tổng quát hợp chất : CnH2n + 2
\(\Rightarrow\) Phản ứng cộng Cl
C3H8 + Cl2 \(\Rightarrow C_3H_7Cl+HCl\)

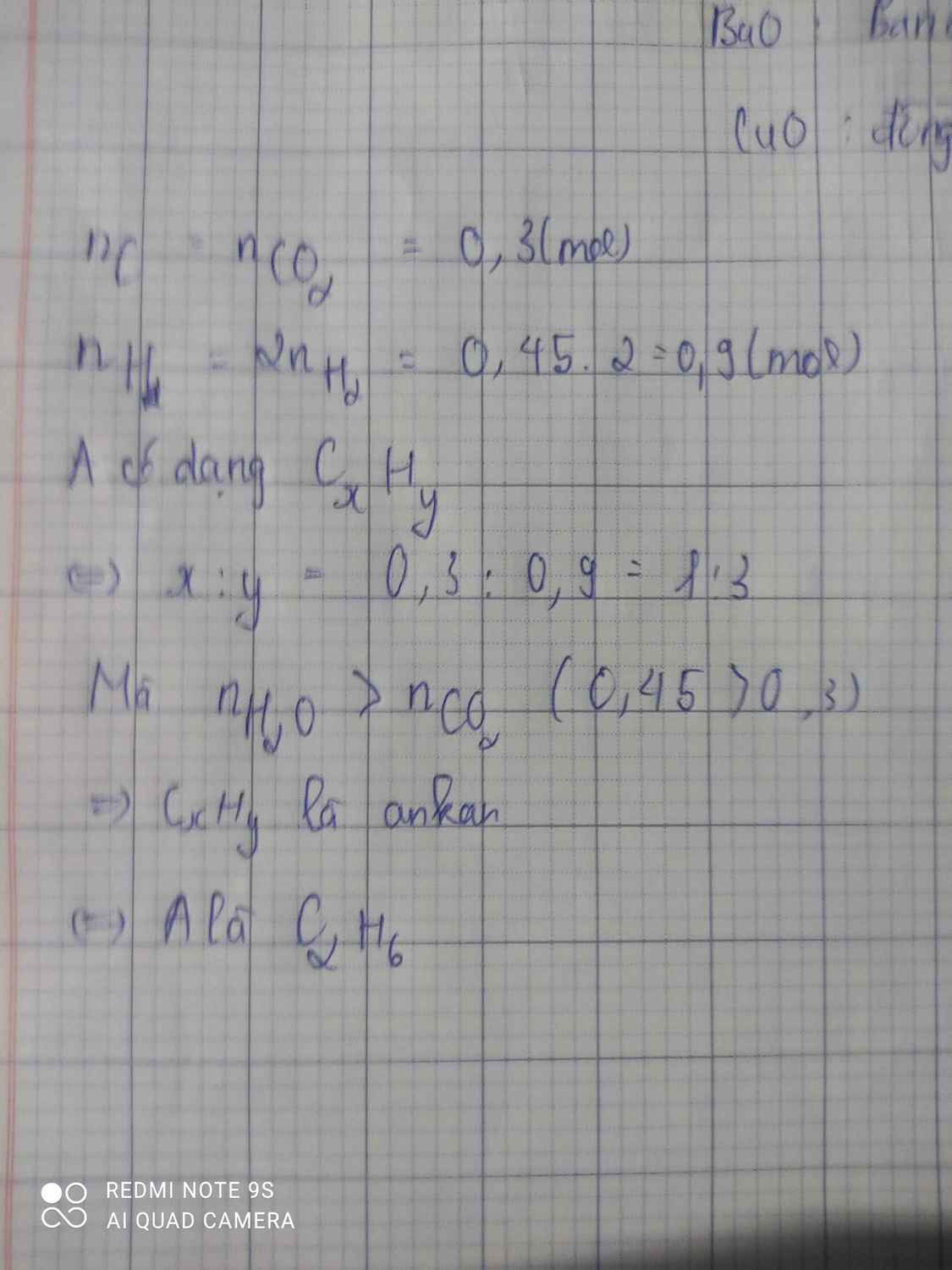






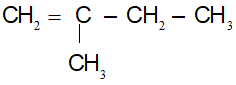
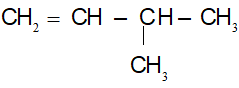
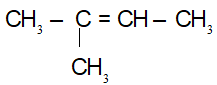

Fe(NO3)3 -> Fe(OH)3 -> Fe2O3 -> Fe
\(Fe\left(NO_3\right)_3+3KOH\rightarrow Fe\left(OH\right)_3\downarrow+3KNO_3\\ 2Fe\left(OH\right)_3\rightarrow\left(t^o\right)Fe_2O_3+3H_2O\\ Fe_2O_3+3CO\rightarrow\left(t^o\right)2Fe+3CO_2\uparrow\)