Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{hh}=\dfrac{3,36}{22,4}=0,15mol\)
\(n_{CO_2}=\dfrac{4,48}{22,4}=0,2mol\)
\(\left\{{}\begin{matrix}n_{CH_4}=x\left(mol\right)\\n_{C_2H_2}=y\left(mol\right)\end{matrix}\right.\)
\(CH_4+2O_2\rightarrow CO_2+2H_2O\)
\(C_2H_2+\dfrac{5}{2}O_2\rightarrow2CO_2+H_2O\)
Từ hai pt trên:\(\Rightarrow\left\{{}\begin{matrix}x+y=0,15\\x+2y=0,2\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,05\end{matrix}\right.\)
\(\%V_{CH_4}=\dfrac{0,1}{0,1+0,05}\cdot100\%=66,67\%\)
\(\%V_{C_2H_2}=100\%-66,67\%=33,33\%\)
\(n_{CO_2}=\dfrac{V_{CO_2}}{22,4}=\dfrac{4,48}{22,4}=0,2mol\)
Gọi \(n_{CH_4}\) là x \(\Rightarrow V_{CH_4}=22,4x\)
\(n_{C_2H_2}\) là y \(\Rightarrow V_{C_2H_2}=22,4y\)
\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
x x ( mol )
\(2C_2H_2+5O_2\rightarrow\left(t^o\right)4CO_2+2H_2O\)
y 2y ( mol )
Ta có:
\(\left\{{}\begin{matrix}22,4x+22,4y=3,36\\x+2y=0,2\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,05\end{matrix}\right.\)
\(\Rightarrow V_{CH_4}=22,4.0,1=2,24l\)
\(\Rightarrow V_{C_2H_2}=22,4.0,05=1,12l\)
\(\%V_{CH_4}=\dfrac{2,24}{3,36}.100=66,67\%\)
\(\%V_{C_2H_2}=100\%-66,67\%=33,33\%\)

a, \(CH_4+2O_2\underrightarrow{^{t^o}}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{^{t^o}}2CO_2+2H_2O\)
b, Gọi: \(\left\{{}\begin{matrix}n_{CH_4}=x\left(mol\right)\\n_{C_2H_4}=y\left(mol\right)\end{matrix}\right.\) \(\Rightarrow x+y=\dfrac{4,48}{22,4}=0,2\left(mol\right)\left(1\right)\)
Theo PT: \(n_{O_2}=2n_{CH_4}+3n_{C_2H_4}=2x+3y=\dfrac{15,68}{22,4}=0,7\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=-0,1\\y=0,3\end{matrix}\right.\)
Đến đây thì ra số mol âm, bạn xem lại đề nhé.

a) Gọi \(\left\{{}\begin{matrix}n_{C_2H_4}=a\left(mol\right)\\n_{C_3H_6}=b\left(mol\right)\end{matrix}\right.\left(a,b>0\right)\Rightarrow a+b=\dfrac{6,72}{22,4}=0,3\left(1\right)\)
PTHH:
\(C_2H_4+3O_2\xrightarrow[]{t^o}2CO_2+2H_2O\)
a-------->3a------>2a
\(2C_3H_6+9O_2\xrightarrow[]{t^o}6CO_2+6H_2O\)
b-------->4,5b---->3b
\(\Rightarrow n_{O_2}=3a+4,5b=\dfrac{23,52}{22,4}=1,05\left(2\right)\)
Từ \(\left(1\right),\left(2\right)\Rightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}\%V_{C_2H_4}=\dfrac{0,2}{0,3}.100\%=66,67\%\\\%V_{C_3H_6}=100\%-66,67\%=33,33\%\end{matrix}\right.\)
b) \(V_{CO_2}=\left(0,2.2+0,1.3\right).22,4=15,68\left(l\right)\)

a.\(n_{hh}=\dfrac{13,44}{22,4}=0,6mol\)
\(n_{C_2H_2Br_4}=\dfrac{6,72}{22,4}=0,3mol\)
\(C_2H_2+2Br_2\rightarrow C_2H_2Br_4\)
0,3 0,3 ( mol )
\(\%C_2H_2=\dfrac{0,3}{0,6}.100=50\%\)
\(\%CH_4=100\%-50\%=50\%\)
b.
\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
0,3 0,6 ( mol )
\(2C_2H_2+5O_2\rightarrow\left(t^o\right)4CO_2+2H_2O\)
0,3 0,75 ( mol )
\(V_{O_2}=\left(0,6+0,75\right).22,4=1,35.22,4=30,24l\)

a)
\(n_{Br_2}=\dfrac{8}{160}=0,05\left(mol\right)\)
PTHH: C2H4 + Br2 --> C2H4Br2
0,05<-0,05
=> \(n_{CH_4}=\dfrac{3,36}{22,4}-0,05=0,1\left(mol\right)\)
\(\%m_{CH_4}=\dfrac{0,1.16}{0,1.16+0,05.28}.100\%=53,33\%\)
\(\%m_{C_2H_4}=\dfrac{0,05.28}{0,1.16+0,05.28}.100\%=46,67\%\)
b)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,1-->0,2
C2H4 + 3O2 --to--> 2CO2 + 2H2O
0,05--->0,15
=> \(V_{O_2}=\left(0,2+0,15\right).22,4=7,84\left(l\right)\)

PT: \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
Giả sử: \(\left\{{}\begin{matrix}n_{CH_4}=x\left(mol\right)\\n_{C_2H_4}=y\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow x+y=\dfrac{7,84}{22,4}=0,35\left(1\right)\)
Ta có: \(n_{O_2}=\dfrac{19,04}{22,4}=0,85\left(mol\right)\)
Theo PT: \(n_{O_2}=2n_{CH_4}+3n_{C_2H_4}=2x+3y\)
⇒ 2x + 3y = 0,85 (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,2\left(mol\right)\\y=0,15\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{CH_4}=\dfrac{0,2.16}{0,2.16+0,15.28}.100\%\approx43,2\%\\\%m_{C_2H_4}\approx56,8\%\end{matrix}\right.\)
Bạn tham khảo nhé!
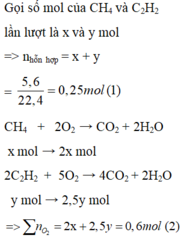

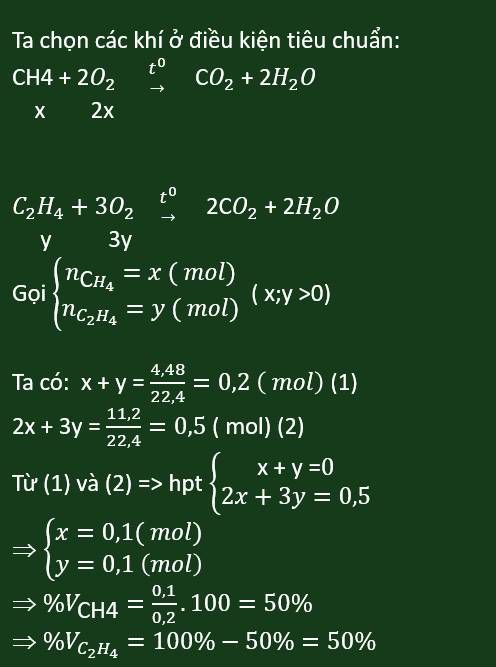
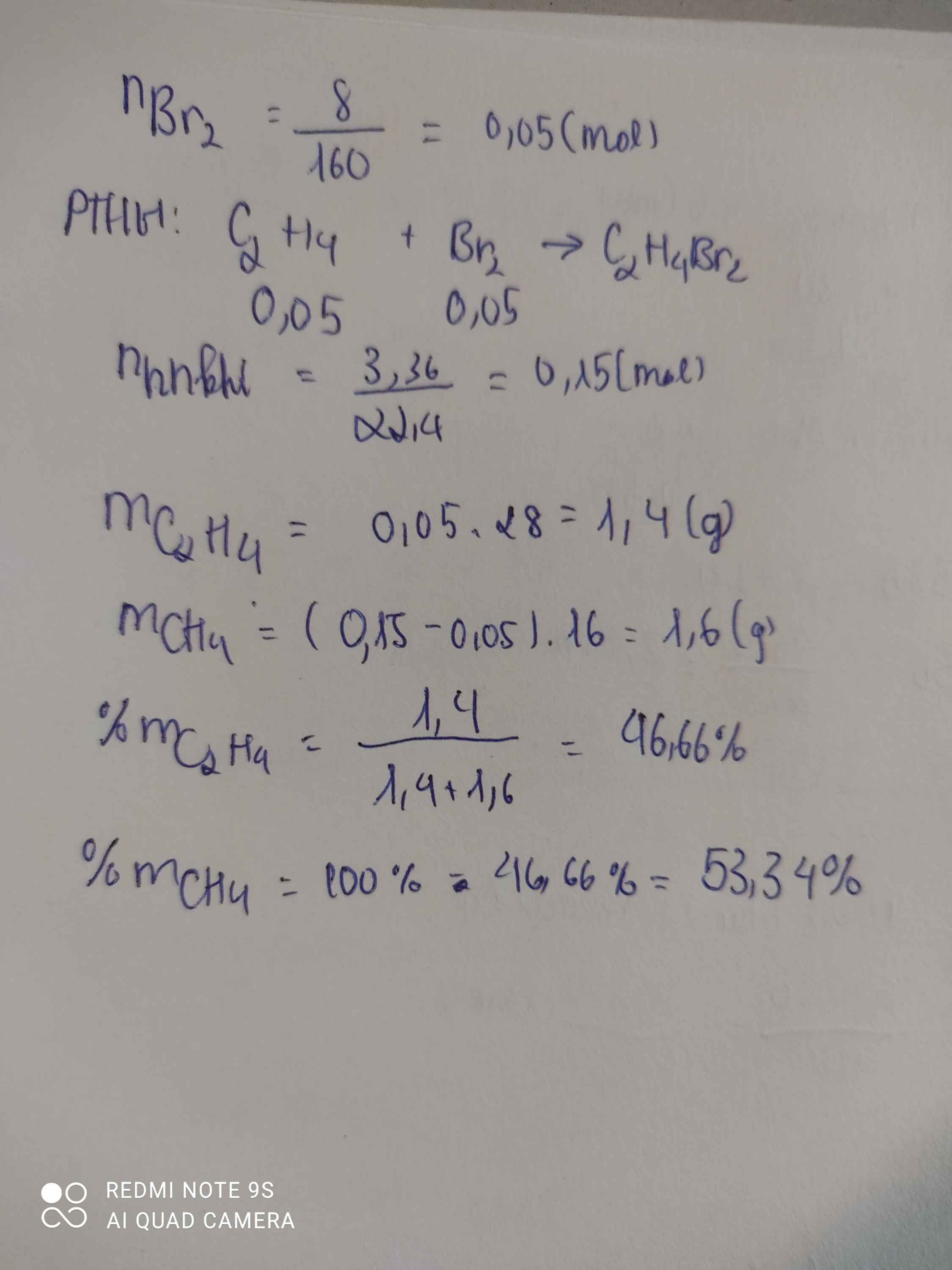
\(V_{CH_4} = a(lít) ; V_{C_2H_2} = b(lít) \Rightarrow a + b = 3,36(1)\\ CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O\\ 2C_2H_2 + 5O_2 \xrightarrow{t^o} 4CO_2 + 2H_2O\\ V_{O_2} = 2a + \dfrac{5}{2}b = 15,68(2)\\ (1)(2) \Rightarrow a = -14,56<0\)
(Sai đề)