Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 1 :
Ta có : \(\left\{{}\begin{matrix}m_{H2SO4}=39,2\\m_{HNO3}=12,6\end{matrix}\right.\) \(\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{H2SO4}=\dfrac{m}{M}=0,4\\n_{HNO3}=\dfrac{m}{M}=0,2\end{matrix}\right.\) \(\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\left\{{}\begin{matrix}n_H=2.0,4=0,8\\n_S=0,4.1=0,4\\n_O=4.0,4=1,6\end{matrix}\right.\\\left\{{}\begin{matrix}n_H=0,2.1=0,2\\n_N=0,2.1=0,2\\n_O=0,2.3=0,6\end{matrix}\right.\end{matrix}\right.\) ( mol )
\(\Rightarrow\left\{{}\begin{matrix}n_O=1,6+0,6=2,2\\n_N=0,2\\n_S=0,4\end{matrix}\right.\) ( mol )
Vậy ....
Bài 2 :
\(CH_4+2O_2\rightarrow CO_2+2H_2O\)
\(C_2H_6+\dfrac{7}{2}O_2\rightarrow2CO_2+3H_2O\)
\(TheoPTHH:n_{O2}=2n_{CH4}+\dfrac{7}{2}n_{C2H6}=1,25\left(mol\right)\)
\(\Rightarrow V=n.22,4=28\left(l\right)\)

\(n_{hh}=\dfrac{11.2}{22.4}=0.5\left(mol\right)\)
\(n_{CH_4}=a\left(mol\right),n_{H_2}=b\left(mol\right)\)
\(\Rightarrow a+b=0.5\left(1\right)\)
\(n_{H_2O}=2a+b=\dfrac{12.6}{18}=0.7\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.2,b=0.3\)
\(n_{CO_2}=n_{CH_4}=0.2\left(mol\right)\)
\(V=0.2\cdot22.4=4.48\left(l\right)\)

\(n_{CaCO_3}=\dfrac{25}{100}=0.25\left(mol\right)\)
\(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
\(0.25...........0.25...........0.25\)
\(C_{M_{Ca\left(OH\right)_2}}=\dfrac{0.25}{0.1}=2.5\left(M\right)\)
\(CH_4+2O_2\underrightarrow{t^0}CO_2+2H_2O\)
\(0.25..............0.25\)
\(V_{CH_4}=0.25\cdot22.4=5.6\left(l\right)\)
a) nCaCO3=0,25(mol)
CH4 + 2 O2 -to-> CO2 + 2 H2O
0,25<------------------0,25(mol)
CO2 + Ca(OH)2 -> CaCO3 + H2O
0,25<------0,25-----------0,25(mol)
b) CMddCa(OH)2= 0,25/0,1= 2,5(M)
b) V(CH4,đktc)=0,25.22,4=5,6(l)

a) \(n_{Na}=\dfrac{2,3}{23}=0,1\left(mol\right)\)
PTHH: 4Na + O2 --to--> 2Na2O
______0,1->0,025----->0,05
=> VO2 = 0,025.22,4 = 0,56(l)
b)
PTHH: Na2O + H2O --> 2NaOH
______0,05----------->0,1
=> \(C_{M\left(NaOH\right)}=\dfrac{0,1}{0,2}=0,5M\)

Có: \(\dfrac{M_A}{M_{O_2}}=\dfrac{\dfrac{m_A}{n_A}}{\dfrac{m_{O_2}}{n_{O_2}}}=\dfrac{m_A}{m_{O_2}}.\dfrac{n_{O_2}}{n_A}=1.\dfrac{V_{O_2}}{V_A}=\dfrac{1}{2}\)
=> \(M_A=\dfrac{1}{2}.M_{O_2}=16\left(g/mol\right)\)
=> A là CH4
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
1---->2
=> VO2 = 2.22,4 = 44,8 (l)
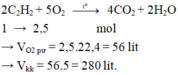
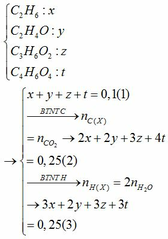
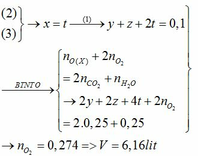
Do đốt cháy A sinh ra sản phẩm chứa các nguyên tố C, H, O; A gồm 2 nguyên tố
=> A gồm C, H
\(\left\{{}\begin{matrix}n_{CO_2}=2\left(mol\right)\\n_{H_2O}=2\left(mol\right)\end{matrix}\right.\)
Bảo toàn O: \(2.n_{O_2}=2.n_{CO_2}+n_{H_2O}\)
=> nO2 = 3 (mol)
=> VO2 = 3.22,4 = 67,2 (l)