Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a.C_2H_5OH+3O_2-^{t^o}\rightarrow2CO_2+3H_2O\\ n_{C_2H_5OH}=0,3\left(mol\right)\\ n_{CO_2}=2n_{C_2H_5OH}=0,6\left(mol\right)\\ \Rightarrow V_{CO_2}=0,6.22,4=13,44\left(l\right)\\ b.n_{O_2}=3n_{C_2H_5OH}=0,6\left(mol\right)\\ MàV_{O_2}=\dfrac{1}{5}V_{kk}\\ \Rightarrow V_{kk}=V_{O_2}.5=0,6.22,4.5=67,2\left(l\right)\\ c.n_{NaOH}=0,9\left(mol\right)\\ Tacó:\dfrac{n_{NaOH}}{n_{CO_2}}=\dfrac{0,9}{0,6}=1,5\\ \Rightarrow Tạora2muốiNaHCO_3vàNa_2CO_3\\ Đặt:n_{NaHCO_3}=x\left(mol\right);n_{Na_2CO_3}=y\left(mol\right)\\ \Rightarrow\left\{{}\begin{matrix}x+y=0,6\left(BTnguyento\left(C\right)\right)\\x+2y=0,9\left(BTnguyento\left(Na\right)\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=0,3\\y=0,3\end{matrix}\right.\\ \Rightarrow m_{muối}=0,3.84+0,3.106=57\left(g\right)\)

a) $C_2H_5OH + 3O_2 \xrightarrow{t^o} 2CO_2 + 3H_2O$
b) $n_{C_2H_5OH} = \dfrac{4,6}{46} = 0,1(mol)$
$n_{O_2} = 3n_{C_2H_5OH} = 0,3(mol)$
$V_{O_2} = 0,3.22,4 = 6,72(lít)$
c)
Theo PTHH :
$n_{CO_2} = 2n_{C_2H_5OH} = 0,2(mol) \Rightarrow V_{CO_2} = 0,2.22,4 = 4,48(lít)$
$n_{H_2O} = 3n_{C_2H_5OH} = 0,3(mol) \Rightarrow m_{H_2O} = 0,3.18 = 5,4(gam)$

a, \(n_{C_2H_6O}=\dfrac{92}{46}=2\left(mol\right)\)
PT: \(C_2H_6O+3O_2\underrightarrow{t^o}2CO_2+3H_2O\)
Theo PT: \(n_{O_2}=3n_{C_2H_6O}=6\left(mol\right)\Rightarrow V_{O_2}=6.22,4=134,4\left(l\right)\)
b, \(V_{kk}=5V_{O_2}=672\left(l\right)\)

a, \(n_{O_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PT: \(C_2H_6O+3O_2\underrightarrow{t^o}2CO_2+3H_2O\)
Theo PT: \(n_{CO_2}=\dfrac{2}{3}n_{O_2}=\dfrac{2}{15}\left(mol\right)\Rightarrow V_{CO_2}=\dfrac{2}{15}.22,4=\dfrac{224}{75}\left(l\right)\)
b, \(n_{C_2H_6O\left(LT\right)}=\dfrac{1}{3}n_{O_2}=\dfrac{1}{15}\left(mol\right)\)
Mà: H = 90%
\(\Rightarrow n_{C_2H_6O\left(TT\right)}=\dfrac{\dfrac{1}{15}}{90\%}=\dfrac{2}{27}\left(mol\right)\)
\(\Rightarrow m_{C_2H_6O}=\dfrac{2}{27}.46=\dfrac{92}{27}\left(g\right)\)

a, PT: \(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
Ta có: \(n_{C_2H_4}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
Theo PT: \(n_{O_2}=3n_{C_2H_4}=0,75\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,75.2,24=16,8\left(l\right)\)
\(\Rightarrow V_{kk}=16,8.5=84\left(l\right)\)
b, Theo PT: \(n_{CO_2}=2n_{C_2H_4}=0,5\left(mol\right)\)
PT: \(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_{3\downarrow}+H_2O\)
Theo PT: \(n_{Ca\left(OH\right)_2}=n_{CaCO_3}=n_{CO_2}=0,5\left(mol\right)\)
\(\Rightarrow m_{\downarrow}=m_{CaCO_3}=0,5.100=50\left(g\right)\)
\(m_{Ca\left(OH\right)_2}=0,5.74=37\left(g\right)\)
\(\Rightarrow m_{ddCa\left(OH\right)_2}=\dfrac{37.100}{2}=1850\left(g\right)\)
Bạn tham khảo nhé!

a, \(C_2H_6O+3O_2\underrightarrow{t^o}2CO_2+3H_2O\)
b, \(n_{CO_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{3}{2}n_{CO_2}=0,3\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,3.22,4=6,72\left(l\right)\)
c, \(n_{C_2H_6O}=\dfrac{1}{2}n_{CO_2}=0,1\left(mol\right)\)
\(\Rightarrow m_{C_2H_6O}=0,1.46=4,6\left(g\right)\)
\(\Rightarrow V_{C_2H_6O}=\dfrac{4,6}{0,8}=5,75\left(ml\right)\)
Độ rượu = \(\dfrac{5,75}{50}.100=11,5^o\)
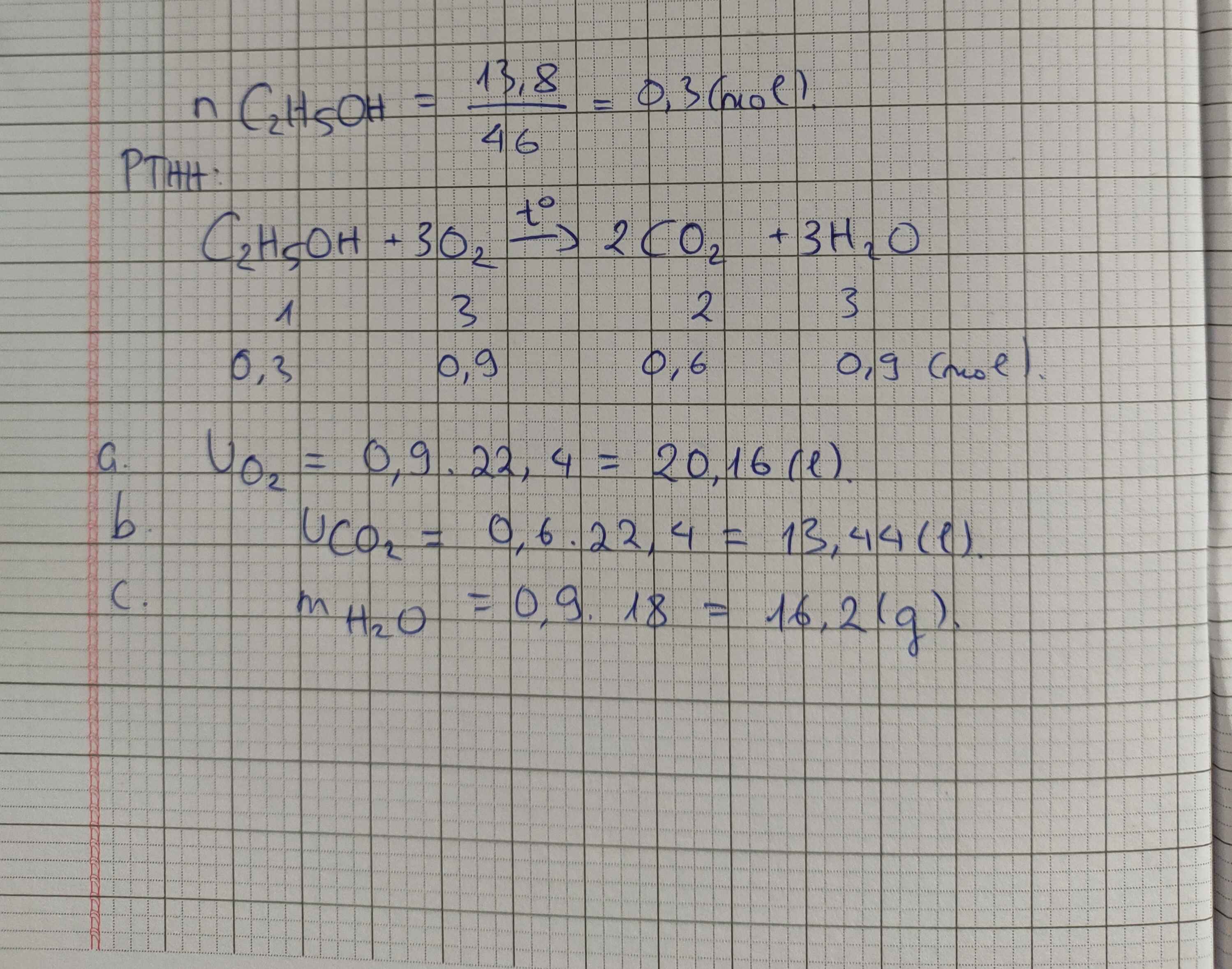
PTHH: \(C_2H_5OH+3O_2\xrightarrow[]{t^o}2CO_2+3H_2O\)
Ta có: \(n_{C_2H_5OH}=\dfrac{6,4}{46}=\dfrac{16}{115}\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{O_2}=\dfrac{48}{115}\left(mol\right)\\n_{CO_2}=\dfrac{32}{112}\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{O_2}=\dfrac{48}{115}\cdot22,4\approx9,35\left(l\right)\\V_{CO_2}=\dfrac{32}{112}\cdot22,4\approx6,23\left(l\right)\end{matrix}\right.\)