Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{hhk}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
\(n_{C_2H_4Br_2}=\dfrac{47}{188}=0,25\left(mol\right)\)
\(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
0,25 0,25 0,25 ( mol )
\(\%V_{C_2H_4}=\dfrac{0,25}{0,4}.100=62,5\%\)
\(\%V_{CH_4}=100-62,5=37,5\%\)
\(V_{Br_2}=\dfrac{0,25}{1}=0,25\left(l\right)\)

a.b.\(m_{tăng}=m_{C_2H_4}=2,8g\)
\(n_{C_2H_4}=\dfrac{2,8}{28}=0,1mol\)
\(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
\(n_{hh}=\dfrac{6,72}{22,4}=0,3mol\)
\(\rightarrow m_{CH_4}=\left(0,3-0,1\right).16=3,2g\)
\(\rightarrow\left\{{}\begin{matrix}\%m_{C_2H_4}=\dfrac{2,8}{2,8+3,2}.100=46,67\%\\\%m_{CH_4}=100\%-46,67\%=53,33\%\end{matrix}\right.\)
c.\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
0,2 0,2 ( mol )
\(C_2H_4+3O_2\rightarrow\left(t^o\right)2CO_2+2H_2O\)
0,1 0,2 ( mol )
\(V_{CO_2}=\left(0,2+0,2\right).22,4=8,96l\)

Ta có: \(n_{Br_2}=\dfrac{4}{160}=0,025\left(mol\right)\)
PT: \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
Theo PT: \(n_{C_2H_4}=n_{Br_2}=0,025\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%V_{C_2H_4}=\dfrac{0,025.22,4}{5,6}.100\%=10\%\\\%V_{CH_4}=90\%\end{matrix}\right.\)

\(n_{CO_2}=\dfrac{8,96}{22,4}=0,4mol\)
\(m_{tăng}=m_{Br_2}=m_{C_2H_2}=2,6g\)
\(\Rightarrow n_{C_2H_2}=\dfrac{2,6}{26}=0,1mol\)
\(C_2H_2+2Br_2\rightarrow C_2H_2Br_4\)
0,1 0,1
\(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(C_2H_2+\dfrac{5}{2}O_2\underrightarrow{t^o}2CO_2+H_2O\)
0,1 0,25 0,2
\(\Rightarrow n_{CO_2\left(CH_4\right)}=0,4-0,2=0,2mol\)
\(\Rightarrow n_{CH_4}=0,2mol\Rightarrow n_{O_2}=0,4mol\)
a)\(\%V_{CH_4}=\dfrac{0,2}{0,4}\cdot100\%=50\%\)
\(\%V_{C_2H_2}=100\%-50\%=50\%\)
b)\(\Sigma n_{O_2}=0,4+0,25=0,65mol\)
\(\Rightarrow V_{O_2}=0,65\cdot22,4=14,56l\)
\(\Rightarrow V_{kk}=14,56\cdot5=72,8l\)

Theo gt ta có: $n_{hh}=0,08(mol);n_{Br_2}=0,08(mol)$
$C_2H_2+2Br_2\rightarrow C_2H_2Br_4$
Suy ra $n_{C_2H_2}=0,04(mol)=n_{CH_4}$
a, $\Rightarrow \%V_{C_2H_2}=\%V_{C_2H_4}=50\%$
b, $CH_4+2O_2\rightarrow CO_2+2H_2O$
$2C_2H_2+5O_2\rightarrow 4CO_2+2H_2O$
Ta có: $n_{O_2}=0,04.2+0,04.5=0,28(mol)\Rightarrow m_{O_2}=8,96(g)$
\(a)C_2H_2 +2Br_2 \to C_2H_2Br_2\\ n_{C_2H_2} = \dfrac{1}{2}n_{Br_2} = \dfrac{0,4.0,2}{2} = 0,04(mol)\\ \Rightarrow V_{C_2H_2} = 0,04.22,4 = 0,896(lít)\\ \%V_{C_2H_2} =\dfrac{0,896}{1,792}.100\% = 50\%\\ \Rightarrow \%V_{CH_4} = 100\% -50\% = 50\%\\ b)\\V_{CH_4} = V_{C_2H_2} = 0,896(lít)\\ CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O\\ C_2H_2 + \dfrac{5}{2}O_2 \xrightarrow{t^o} 2CO_2 + H_2O\\ \)
\(V_{O_2} = 2V_{CH_4} + \dfrac{5}{2}V_{C_2H_2} = 4,032(lít)\\ \Rightarrow m_{O_2} = \dfrac{4,032}{22,4}.32 = 5,76(gam)\)
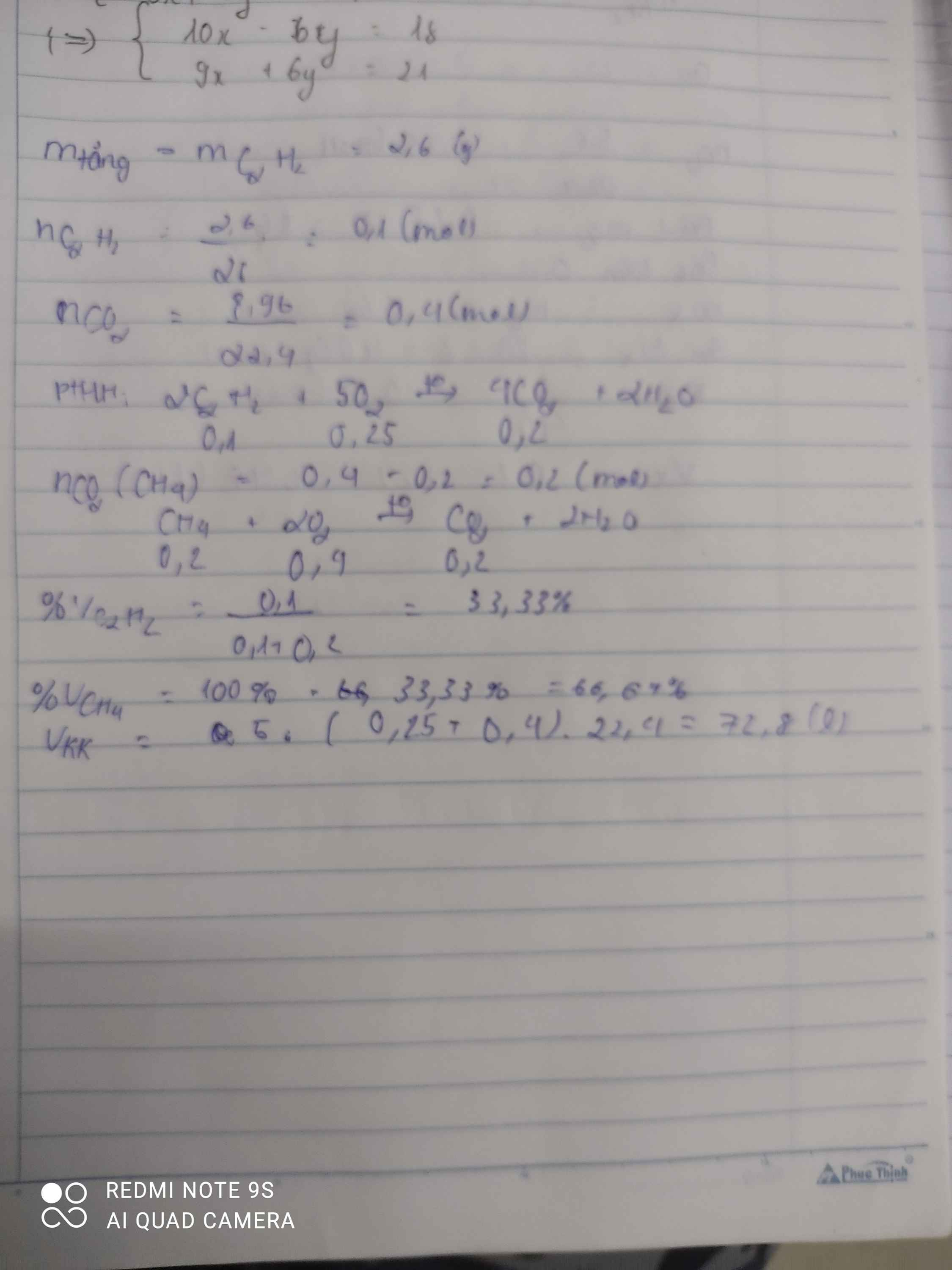

C2H4+Br2->C2H4Br2
n C2H4=\(\dfrac{1,4}{28}\)=0,05 mol
=>VC2H4=0,05\(\dfrac{22,4}{4,48}.100\)=25%
=>VCH4=100-25=75%