Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bảo toàn KL: \(m_{FeCl_3}+m_{KOH}=m_{Fe\left(OH\right)_3}+m_{KCl}\)
\(\Rightarrow m_{FeCl_3}=7+8,25-5,5=9,75\left(g\right)\)
Chọn D

Sơ đồ
Sắt (III) sunfat + Natri hidroxit → Sắt (III) hidroxit + natri sunfat
Áp dụng ĐLBTKL, ta có
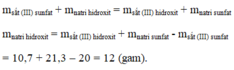

\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\\ 2Fe+3Cl_2\rightarrow\left(t^o\right)2FeCl_3\\ n_{Cl_2}=\dfrac{3}{2}.0,2=0,3\left(mol\right)\\ n_{FeCl_3}=n_{Fe}=0,2\left(mol\right)\\ a,V_{Cl_2\left(đktc\right)}=0,3.22,4=6,72\left(l\right)\\ b,m_{FeCl_3}=162,5.0,2=32,5\left(g\right)\)
a, \(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
PT: \(2Fe+3Cl_2\underrightarrow{t^o}2FeCl_3\)
Theo PT: \(n_{Cl_2}=\dfrac{3}{2}n_{Fe}=0,3\left(mol\right)\)
\(\Rightarrow V_{Cl_2}=0,3.22,4=6,72\left(l\right)\)
b, \(n_{FeCl_3}=n_{Fe}=0,2\left(mol\right)\Rightarrow m_{FeCl_3}=0,2.162,5=32,5\left(g\right)\)

\(n_{Fe}=\dfrac{5.6}{56}=0.1\left(mol\right)\)
\(Fe+\dfrac{3}{2}Cl_2\underrightarrow{^{^{t^0}}}FeCl_3\)
\(0.1.......0.15..........0.1\)
\(V_{Cl_2}=0.15\cdot22.4=3.36\left(l\right)\)
\(m_{FeCl_3}=0.1\cdot162.5=16.25\left(g\right)\)

Bài 1 :
a) Pt : 2Ba + O2 → (to) 2BaO
b) Pt : 2Fe(OH)3 + 3H2SO4 → Fe2(SO4)3 + 6H2O
c) Pt : ZnCl2 + 2NaOH → Zn(OH)2 + 2NaCl
d) Pt : Na2CO3 + 2HCl → 2NaCl + CO2 + H2O
Chúc bạn học tốt

\(13,\\ PTHH:2Fe\left(OH\right)_3\rightarrow^{t^o}Fe_2O_3+3H_2O\\ \text{Bảo toàn KL: }m_{Fe\left(OH\right)_3}=m_{Fe_2O_3}+m_{H_2}\\ \Rightarrow m_{Fe\left(OH\right)_3\left(\text{p/ứ}\right)}=13,5+40=53,5\left(g\right)\\ \Rightarrow\%_{Fe\left(OH\right)_3\left(\text{phân hủy}\right)}=\dfrac{53,5}{100}\cdot100\%=53,5\%\)

a.Theo ĐLVBTKL mFeCl3 + mNaOH = mFe(OH)3 + mNaCl
b.=> mFe(OH)3 = (mFeCl3 + mNaOH)-mNaClto
mFe(OH)3 = (16,25+12)-17,55 = 10,7 g

\(2KClO_3\rightarrow3O_2+2KCl\)
\(m_{KClO_3}=m_{O_2}+m_{KCl}\)
\(\Rightarrow m_{KCl}=m_{KClO_3}-m_{KCl}=24,5-9,6=14,9\left(g\right)\)

BT1 :
Bảo toàn khối lượng :
\(m_{FeCl_3}=m_{Fe}+m_{Cl_2}=11.2+21.3=32.5\left(g\right)\)
\(Fe+\dfrac{3}{2}Cl_2\underrightarrow{^{^{t^0}}}FeCl_3\)
\(1.5.....2.25......1.5\)
\(n_{Fe}=\dfrac{9\cdot10^{23}}{6\cdot10^{23}}=1.5\left(mol\right)\)
Số phân tử Cl2 : \(2.25\cdot6\cdot10^{23}=13.5\cdot10^{23}\left(pt\right)\)
Số phân tử FeCl3 : \(1.5\cdot6\cdot10^{23}=9\cdot10^{23}\left(pt\right)\)
a) PTHH: FeCl3 + 3KOH → Fe(OH)3 + 3KCl
b) Theo ĐLBTKL ta có:
\(m_{FeCl_3}+m_{KOH}=m_{Fe\left(OH\right)_3}+m_{KCl}\)
\(\Leftrightarrow m_{FeCl_3}=m_{Fe\left(OH\right)_3}+m_{KCl}-m_{KOH}=2,14+4,47-3,36=3,25\left(g\right)\)