Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\\ Mg+2HCl\rightarrow MgCl_2+H_2\\ 0,2..............0,4.............0,2...............0,2\left(mol\right)\\ a,V_{H_2\left(đktc\right)}=0,2.22,4=4,48\left(l\right)\\ b,m_{MgCl_2}=95.0,2=19\left(g\right)\\ c,a=C_{MddHCl}=\dfrac{0,4}{0,2}=2\left(M\right)\)

MnO 2 + HCl → MnCl 2 + Cl 2 + 2 H 2 O
Cl 2 + 2NaOH → NaCl + NaClO + H 2 O
n MnO 2 = 0,2 mol; n NaOH = 0,729 mol
Theo phương trình (1) ta có: n Cl 2 = n MnO 2 = 0,2 mol
Theo phương trình (2) ta có: 2 n Cl 2 < n NaOH ⇒ NaOH dư
Dung dịch A gồm: n NaCl = n NaClO = n Cl 2 = 0,2 mol
n NaOH dư = 0,729 – 2.0,2 = 0,329 mol
m dd A = m Cl 2 + m dd NaOH = 0,2.71 + 145,8 = 160g
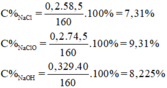

1/ Gọi x, y lần lượt là số mol của Na2CO3 và KHCO3.
Khi cho từ từ HCl vào dung dịch A thì các phản ứng xảy ra lần lượt là :
CO3^2- + H^+ => HCO3-
x ---------> x ----------> x
HCO3^- + H+ => H2O + CO2.
0,045 <--- 0,045 <-------- 0,045
.........HCO3^- + OH- => CO3^2- + H2O.
x+y - 0,045 -------------> x+y-0,045.
Giải hệ: x+y-0,045 = 29,55/197; n HCl = x+ 0,045 = 0,15.
=> x = 0,105 ; y = 0,09.
2/ Nồng độ của HCO3- , CO3^2- lần lượt là 0,225 M; 0,2625 M.
3/ Cho từ từ dung dịch A vào bình đựng 100 ml dung dịch HCl 1,5 M => Các phản ứng xảy ra đồng thời:
CO3^2- + 2 H^+ => H2O + CO2.
HCO3- + H+ => H2O + CO2.
Do tỉ lệ trong hỗn hợp : n CO3^2-/ n HCO3- = 7/6 => 7x*2+6x = 0,15 => x=0,0075.
=> V = 2,184 lít.

\(n_{KMnO_4}=\frac{15,8}{158}=0,1\left(mol\right)\)
PTHH : \(2KMnO_4+16HCl-->2KCl+2MnCl_2+5Cl_2+8H_2O\) (1)
\(Cl_2+H_2-as->2HCl\) (2)
Có : \(m_{ddHCl}=100\cdot1,05=105\left(g\right)\)
=> \(m_{HCl}=105-97,7=7,3\left(g\right)\)
=> \(n_{HCl}=\frac{7,3}{36,5}=0,2\left(mol\right)\)
BT Clo : \(n_{Cl_2}=\frac{1}{2}n_{HCl}=0,1\left(mol\right)\)
Mà theo lí thuyết : \(n_{Cl_2}=\frac{5}{2}n_{KMnO_4}=0,25\left(mol\right)\)
=> \(H\%=\frac{0,1}{0,25}\cdot100\%=40\%\)
Vì spu nổ thu được hh hai chất khí => \(\hept{\begin{cases}H_2\\HCl\end{cases}}\) (Vì H2 dư)
=> \(n_{hh}=\frac{13,44}{22,4}=0,6\left(mol\right)\)
=> \(n_{H_2\left(spu\right)}=n_{hh}-n_{HCl\left(spu\right)}=0,6-0,2=0,4\left(mol\right)\)
BT Hidro : \(\Sigma_{n_{H2\left(trong.binh\right)}}=n_{H_2\left(spu\right)}+\frac{1}{2}n_{HCl}=0,4+0,1=0,5\left(mol\right)\)
đọc thiếu đề câu a wtf
\(C_{M\left(HCl\right)}=\frac{0,2}{0,1}=2\left(M\right)\)

a) CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
b) \(n_{CaCO_3}=\dfrac{10}{100}=0,1\left(mol\right)\)
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
_0,1---->0,2------->0,1----->0,1
=> mCaCl2 = 0,1.111 = 11,1 (g)
=> VCO2 = 0,1.22,4 = 2,24 (l)
c) \(a=C_{M\left(HCl\right)}=\dfrac{0,2}{0,4}=0,5M\)
d) \(C_{M\left(CaCl_2\right)}=\dfrac{0,1}{0,4}=0,25M\)

\(a.BTNT\left(H\right):n_{HCl}=2n_{H_2}=0,65\left(mol\right)\\ \Rightarrow CM_{HCl}=\dfrac{0,65}{0,5}=1,3M\\ b.2Al+6HCl\rightarrow2AlCl_3+3H_2\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ Đặt:\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}\dfrac{3}{2}x+y=0,325\\27x+56y=9,65\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=0,15\\y=0,1\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{Al}=4,05\left(g\right)\\m_{Fe}=5,6\left(g\right)\end{matrix}\right.\)

a) 2Al + 6HCl -> 2AlCl3 + 3H2
Al2O3 + 6HCl -> 2AlCl3 + 3H2O
nH2 = 0,15mol => nAl=0,1mol => mAl=2,7g; mAl2O3 = 10,2g => nAl2O3 = 0,1mol
=>%mAl=20,93% =>%mAl2O3 = 79,07%
b) nHCl = 0,1.3+0,1.6=0,9 mol=>mHCl(dd)=100g
mddY=12,9+100-0,15.2=112,6g
mAlCl3=22,5g=>C%=19,98%

a)
$M + 2HCl \to MCl_2 + H_2$
$n_{HCl} = 0,3.1 = 0,3(mol)$
Theo PTHH : $n_M = \dfrac{1}{2}n_{HCl} = 0,15(mol)$
$\Rightarrow M = \dfrac{3,6}{0,15} = 24(Mg)$
b)
$n_{MgCl_2} = n_{Mg} = 0,15(mol)$
$m_{MgCl_2} = 0,15.95 = 14,25(gam)$
c) $n_{H_2} = n_{Mg} = 0,15(mol)$
$V_{H_2} = 0,15.22,4 = 3,36(lít)$
\(n_{Cl_2}=\dfrac{6,1975}{24,79}=0,25mol\\ 2KMnO_4+16HCl\xrightarrow[]{}2KCl+2MnO_2+5Cl_2+8H_2O\)
0,1 0,8 0,1 0,1 0,25 0,4
\(m=m_{KMnO_4}=0,1.158=15,8g\\ a=C_{M_{HCl}}=\dfrac{0,8}{0,1}=8M\\ b)n_{NaOH}=0,3.1=0,3mol\\ 2NaOH+Cl_2\rightarrow NaCl+NaClO+H_2O\\ \Rightarrow\dfrac{0,3}{2}< \dfrac{0,25}{1}=>Cl_2.dư\\ n_{NaCl}=n_{NaClO}=\dfrac{1}{2}n_{NaOH}=0,15mol\\ C_{M_{NaCl}=}C_{M_{NaClO}}=\dfrac{0,15}{0,3}=0,5M\)