Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(n_{CH_3COOH}=0,2.1=0,2\left(mol\right)\)
PT: \(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
Theo PT: \(n_{Mg}=n_{H_2}=\dfrac{1}{2}n_{CH_3COOH}=0,1\left(mol\right)\)
\(\Rightarrow m=m_{Mg}=0,1.24=2,4\left(g\right)\)
\(V=V_{H_2}=0,1.22,4=2,24\left(l\right)\)
b, \(C_2H_5OH+O_2\underrightarrow{^{mengiam}}CH_3COOH+H_2O\)
Theo PT: \(n_{C_2H_5OH}=n_{CH_3COOH}=0,2\left(mol\right)\)
\(\Rightarrow m_{C_2H_5OH}=0,2.46=9,2\left(g\right)\)
\(\Rightarrow V_{ddC_2H_5OH}=\dfrac{9,2}{0,8}=11,5\left(ml\right)\)

a) PTHH: \(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\uparrow\)
Ta có: \(n_{CH_3COOH}=0,2\cdot1=0,2\left(mol\right)\)
\(\Rightarrow n_{Mg}=n_{H_2}=0,1\left(mol\right)\) \(\Rightarrow\left\{{}\begin{matrix}m_{Mg}=0,1\cdot24=2,4\left(g\right)\\V_{H_2}=0,1\cdot22,4=2,24\left(l\right)\end{matrix}\right.\)
b) PTHH: \(C_2H_5OH+O_2\xrightarrow[]{men}CH_3COOH+H_2O\)
Theo PTHH: \(n_{C_2H_5OH}=n_{CH_3COOH}=0,2\left(mol\right)\)
\(\Rightarrow m_{ddC_2H_5OH}=\dfrac{0,2\cdot46}{8\%}=115\left(g\right)\) \(\Rightarrow V_{C_2H_5OH}=\dfrac{115}{0,8}=143,75\left(ml\right)\)

\(a)n_{CH_3COOH} = 0,2.2 = 0,4(mol)\\ Mg + 2CH_3COOH \to (CH_3COO)_2Mg + H_2\\ n_{Mg} = \dfrac{1}{2}n_{CH_3COOH} = 0,2(mol)\\ m_{Mg} = 0,2.24 = 4,8(gam)\\ b)\\ CH_3COOH + C_2H_5OH \buildrel{{H_2SO_4}}\over\rightleftharpoons CH_3COOC_2H_5 + H_2O\\ n_{CH_3COOH\ pư} = n_{este} = \dfrac{24,64}{88} = 0,28(mol)\\ H = \dfrac{0,28}{0,4}.100\% = 70\%\)

nKOH = 0,5.0,3 = 0,15 mol
CH3COOH + KOH → CH3COOK + H2O
0,15 0,15 0,15 mol
a) CM CH3COOH = 0,15/0,2 =0,75M
b) Thể tích của dung dịch thu được sau phản ứng: 500 ml
CM CH3COOK = 0,15/0,5 = 0,3M
c) Phản ứng lên men giấm
C2H5OH + O2 → CH3COOH + H2O
0,15 0,15
→ mC2H5OH = 0,15.46 = 6,9 gam
\(n_{KOH}=0,5\cdot0,3=0,15mol\)
\(CH_3COOH+KOH\rightarrow CH_3COOK+H_2O\)
0,15 0,15 0,15 0,15
a)\(C_{M_{CH_3COOH}}=\dfrac{0,15}{0,2}=0,75M\)
b)\(C_{M_{CH_3COOK}}=\dfrac{0,15}{0,2+0,3}=0,3M\)

Ta có: \(n_{HCl}=\dfrac{200}{1000}.2=0,4\left(mol\right)\)
\(PTHH:Mg+2HCl--->MgCl_2+H_2\uparrow\left(1\right)\)
a. Theo PT(1): \(n_{Mg}=n_{H_2}=n_{MgCl_2}=\dfrac{1}{2}.n_{HCl}=\dfrac{1}{2}.0,4=0,2\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Mg}=0,2.24=4,8\left(g\right)\\V_{H_2}=0,2.22,4=4,48\left(lít\right)\end{matrix}\right.\)
b. \(PTHH:2NaOH+MgCl_2--->Mg\left(OH\right)_2\downarrow+2NaCl\left(2\right)\)
Ta có: \(n_{NaOH}=\dfrac{\dfrac{20\%.100}{100\%}}{40}=0,5\left(mol\right)\)
Ta thấy: \(\dfrac{0,5}{2}>\dfrac{0,2}{1}\)
Vậy NaOH dư.
Theo PT(2): \(n_{Mg\left(OH\right)_2}=n_{MgCl_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Mg\left(OH\right)_2}=0,2.58=11,6\left(g\right)\)
a: \(Mg+2HCl\rightarrow MgCl_2+H_2\)
200ml=0,2 lít
\(n_{HCl}=0.2\cdot22.4=4.48\left(mol\right)\)
\(\Leftrightarrow n_{H_2}=2.24\left(mol\right)\)
\(\Leftrightarrow m_{H_2}=n_{H_2}\cdot M=2.24\cdot1=2.24\left(g\right)\)
\(n_{MgCl_2}=2.24\left(mol\right)\)
\(\Leftrightarrow n_{Mg}=2.24\left(mol\right)\)
\(\Leftrightarrow m_{Mg}=2.24\cdot24=53.76\left(g\right)\)

Đáp án: A
Vì dung dịch rượu gồm rượu etylic và nước nên ta gọi:
n H 2 O = x m o l và n C 2 H 5 O H = y m o l
PTHH:
2 N a + 2 H 2 O → 2 N a O H + H 2 ↑ ( 1 )
x mol → 0,5.x mol
2 N a + 2 C 2 H 5 O H → 2 C 2 H 5 O N a + H 2 ↑
y mol → 0,5.y mol
Ta có hệ phương trình:
18 x + 46 y = 10 , 1 0 , 5 x + 0 , 5 y = 0 , 125 ⇒ x = 0 , 05 y = 0 , 2
V C 2 H 5 O H nguyên chất = m D = 0 , 2 . 46 0 , 8 = 11 , 5 m l
V H 2 O = m D = 10 , 1 - 9 , 2 1 = 0 , 9 m l
=> V d d r ư ợ u = V H 2 O + V C 2 H 5 O H = 0,9 + 11,5 = 12,4 ml
=> Độ rượu D 0 = V C 2 H 5 O H V d d r u o u . 100 = 11 , 5 12 , 4 . 100 = 92 , 74 0

a.b.\(n_{Mg}=\dfrac{4,8}{24}=0,2mol\)
\(2Mg+2CH_3COOH\rightarrow2\left(CH_3COO\right)_2Mg+H_2\)
0,2 0,2 0,2 ( mol )
\(C_{M_{CH_3COOH}}=\dfrac{0,2}{0,2}=1M\)
\(m_{\left(CH_3COO\right)_2Mg}=0,2.142=28,4g\)
c.Sửa đề: thu được 9,2g este
\(n_{CH_3COOC_2H_5}=\dfrac{9,2}{88}=0,1mol\)
\(CH_3COOH+C_2H_5OH\rightarrow CH_3COOC_2H_5+H_2O\)
Thực tế: 0,2 0,1 ( mol )
Lý thuyết: 0,1 0,1 ( mol )
\(H=\dfrac{0,1}{0,2}.100=50\%\)

Rượu etylic \(C_2H_5OH\)
Axit axetic \(CH_3COOH\)
\(n_{CO_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(2CH_3COOH+Na_2CO_3\rightarrow2CH_3COONa+H_2O+CO_2\uparrow\)
0,2 0,1 0,2 0,1 0,1
\(\%m_{CH_3COOH}=\dfrac{0,2\cdot60}{39,6}\cdot100\%=30,3\%\)
\(\%m_{C_2H_5OH}=100\%-30,3\%=69,7\%\)
a)
\(n_{CO_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: 2CH3COOH + Na2CO3 --> 2CH3COONa + CO2 + H2O
0,2<----------0,1<-------------0,2<-------0,1
=> \(m_{CH_3COOH}=0,2.60=12\left(g\right)\)
\(\%m_{CH_3COOH}=\dfrac{12}{39,6}.100\%=30,3\%\)
\(\%m_{C_2H_5OH}=\dfrac{39,6-12}{39,6}.100\%=69,7\%\)
b) dd sau pư chứa \(\left\{{}\begin{matrix}CH_3COONa:0,2\left(mol\right)\\C_2H_5OH:\dfrac{39,6-12}{46}=0,6\left(mol\right)\end{matrix}\right.\)
\(V_{dd}=\dfrac{0,1}{2}=0,05\left(l\right)\)
=> \(\left\{{}\begin{matrix}C_{M\left(CH_3COONa\right)}=\dfrac{0,2}{0,05}=4M\\C_{M\left(C_2H_5OH\right)}=\dfrac{0,6}{0,05}=12M\end{matrix}\right.\)
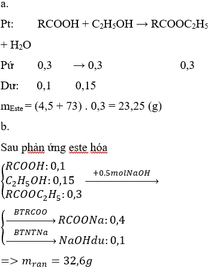
Phương trình hóa học:
Mg + 2CH3COOH => (CH3COO)2Mg + H2
nCH3COOH = CM.V = 0.2 x 1 = 0.2 (mol)
Theo phương trình ==> nMg = 0.1 (mol) => mMg = n.M = 0.1 x 24 = 2.4 (g)
Theo phương trình ==> nH2 = 0.1 (mol) ==> VH2 =22.4 x n = 22.4 x 0.1 = 2.24 (l)
C2H5OH + O2 => (men giấm) CH3COOH + H2O
nCH3COOH = 0.2 (mol) => nC2H5OH = 0.2 (mol)
mC2H5OH = n.M = 0.2 x 46 = 9.2 (g)
V = m/D = 9.2/8 = 1.15ml
nCH3COOH= 0.2 mol
Mg + 2CH3COOH --> (CH3COO)2Mg + H2
Từ PTHH:
nMg= 0.1 mol
mMg=2.4g
nH2= 0.1 mol
VH2= 2.24l
C2H5OH + O2 -mg-> CH3COOH + H2O
0.2___________________0.2
mC2H5OH= 9.2g
VC2H5OH=9.2/0.8=11.5ml
V hhr= 11.5*100/8=143.75ml