Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(n_{CH_4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
\(n_{CO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,05-->0,1------->0,05
2C2H2 + 5O2 --to--> 4CO2 + 2H2O
0,125<--0,3125<----0,25
=> \(\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,05}{0,05+0,125}.100\%=28,57\%\\\%V_{C_2H_2}=\dfrac{0,125}{0,05+0,125}.100\%=71,43\%\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%m_{CH_4}=\dfrac{0,05.16}{0,05.16+0,125.26}.100\%=19,753\%\\\%m_{C_2H_2}=\dfrac{0,125.26}{0,05.16+0,125.26}.100\%=80,247\%\end{matrix}\right.\)
b) \(n_{O_2}=0,1+0,3125=0,4125\left(mol\right)\)
=> \(V_{O_2}=0,4125.22,4=9,24\left(l\right)\)
=> Vkk = 9,24.5 = 46,2 (l)

\(a,Đặt:n_{CH_4}=a\left(mol\right);n_{C_4H_{10}}=b\left(mol\right)\left(a,b>0\right)\\ PTHH:CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\\ 2C_4H_{10}+13O_2\rightarrow\left(t^o\right)8CO_2+10H_2O\\ \Rightarrow\left\{{}\begin{matrix}16a+58b=7,4\\22,4a+22,4.4b=22\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{CH_4}=0,1.16=1,6\left(g\right)\\m_{C_4H_{10}}=0,1.58=5,8\left(g\right)\end{matrix}\right.\\ b,n_{O_2}=2a+\dfrac{13}{2}b=2.0,1+6,5.0,1=0,85\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,85.22,4=19,04\left(l\right)\)

nC = 4,8/12 = 0,4 mol
nS = 6,4/32 = 0,2 mol
a. C + O2 -> (nhiệt độ) CO2
S + O2 -> (nhiệt độ) SO2
nO2 = nC + nS = 0,6 mol
=> nN2 = 4 x nO2 = 2,4 mol
=> n không khí = 3 mol => V = 67,2 L
b. mB = 44 x 0,4 + 64 x 0,2 = 30,4 g
nB = 0,6 mol
=> M(trung bình của B) = 30,4/0,6 = 50,67 g/mol

\(m_{Al}=27,8.19,2\%=5,4\left(g\right)\\ m_{Fe}=27,8-5,4=22,4\left(g\right)\\ \rightarrow\left\{{}\begin{matrix}n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\\n_{Fe}=\dfrac{22,4}{56}=0,4\left(mol\right)\end{matrix}\right.\)
PTHH:
4Al + 3O2 --to--> 2Al2O3
0,2-->0,15------->0,1
3Fe + 2O2 --to--> Fe3O4
0,4-->4/15--------->2/15
\(\rightarrow\left\{{}\begin{matrix}V_{kk}=\left(0,15+\dfrac{4}{15}\right).22,4.5=\dfrac{140}{3}\left(l\right)\\m_{Cran}=0,1.102+\dfrac{2}{15}.232=\dfrac{617}{15}\left(g\right)\end{matrix}\right.\)
mAl=27,8.19,42%=5,4g
⇒nAl=\(\dfrac{5,4}{27}\)=0,2mol
⇒nFe=\(\dfrac{27,8-5,4}{56}\)=0,4mol
4Al+3O2to→2Al2O34
3Fe+2O2to→Fe3O4
⇒nO2=\(\dfrac{3}{4}\)nAl+\(\dfrac{2}{3}\)nFe=\(\dfrac{5}{12}\)mol
⇒Vkk=\(\dfrac{5}{12}\).22,4.5=46,67l
b,
mrắn=27,8+mO2=27,8+\(\dfrac{5}{12}\)32=41,1g

\(M_{hỗn\ hợp} = 4,5.2 = 9\\ Gọi : n_{CH_4} = a(mol) ; n_{H_2} = b(mol)\\ \Rightarrow 16a + 2b =9(a + b)\ (1) n_{O_2} = \dfrac{56}{5.22,4} = 0,5(mol)\\ CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O\\ 2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ n_{O_2} = 2a + 0,5b = 0,5(2)\\ (1)(2) \Rightarrow a = 0,2 ; b = 0,2\\ \Rightarrow V = (0,2 + 0,2).22,4 = 8,96(lít)\)

nO (trong CO2) = 2 . nCO2 = 2 . 26,4/44 = 1,2 (mol)
nO (trong H2O) = nH2O = 13,5/18 = 0,75 (mol)
nO (trong O2) = 1,2 + 0,75 = 1,95 (mol)
nO2 = 1,95/2 = 0,975 (mol)
VO2 = 0,975 . 22,4 = 21,84 (l)
Vkk = 21,84 . 5 = 109,2 (l)
nO (trong CO2) = 2 . nCO2 = 2 . 26,4/44 = 1,2 (mol)
nO (trong H2O) = nH2O = 13,5/18 = 0,75 (mol)
nO (trong O2) = 1,2 + 0,75 = 1,95 (mol)
nO2 = 1,95/2 = 0,975 (mol)
VO2 = 0,975 . 22,4 = 21,84 (l)
Vkk = 21,84 . 5 = 109,2 (l)
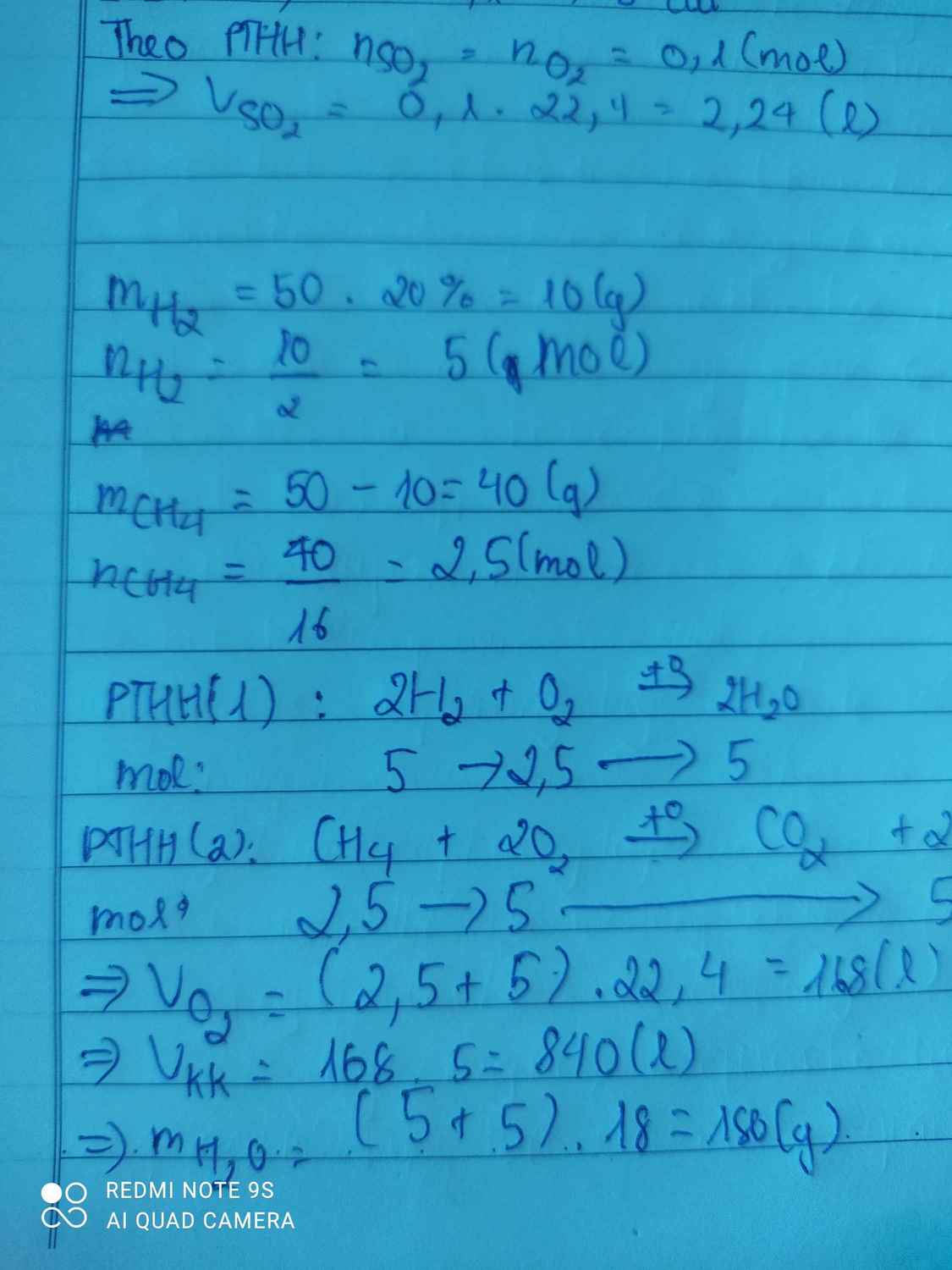
Vì: %mCH4 = 80%
\(\Rightarrow m_{CH_4}=25.80\%=20\left(g\right)\Rightarrow n_{CH_4}=\dfrac{20}{16}=1,25\left(mol\right)\)
\(\Rightarrow m_{H_2}=5\left(g\right)\Rightarrow n_{H_2}=\dfrac{5}{2}=2,5\left(mol\right)\)
PT: \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
\(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
Theo PT: \(\Sigma n_{O_2}=\dfrac{1}{2}n_{H_2}+2n_{CH_4}=3,75\left(mol\right)\)
\(\Rightarrow V_{O_2}=3,75.22,4=84\left(l\right)\)
Mà: %VO2 = 20%
\(\Rightarrow V_{kk}=\dfrac{84}{20\%}=420\left(l\right)\)
Bạn tham khảo nhé!