Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\\ KL:M\\ M+2HCl\rightarrow MCl_2+H_2\\ n_{MCl_2}=n_M=n_{H_2}=0,05\left(mol\right)\\ M_{MCl_2}=\dfrac{4,75}{0,05}=95\left(\dfrac{g}{mol}\right)\\ M\text{à}:M_{MCl_2}=M_M+71\left(\dfrac{g}{mol}\right)\\ \Rightarrow M_M+71=95\\ \Leftrightarrow M_M=24\left(\dfrac{g}{mol}\right)\\ \Rightarrow M\left(II\right):Magie\left(Mg=24\right)\\ a=24.0,05=1,2\left(g\right)\)

\(n_{H_2}=\dfrac{2.24}{22.4}=0.1\left(mol\right)\)
\(R+2HCl\rightarrow RCl_2+H_2\)
\(0.1........0.2................0.1\)
\(M_R=\dfrac{13.7}{0.1}=137\left(\dfrac{g}{mol}\right)\)
\(R:Ba\)
\(200\left(ml\right)=0.2\left(l\right)\)
\(C_{M_{HCl}}=\dfrac{0.2}{0.2}=1\left(M\right)\)

1a)
nH2 = 2.688/22.4 = 0.12 (mol)
M + 2HCl => MCl2 + H2
0.12..............0.12......0.12
MM = 4.8/0.12 = 40
=> M là : Ca
mCaCl2 = 0.12 * 111 = 13.32 (g)

a, \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: M + 2H2O → M(OH)2 + H2
Mol: 0,1 0,1 0,1
\(\Rightarrow M_M=\dfrac{4}{0,1}=40\left(g/mol\right)\)
⇒ M là canxi (Ca)
\(C\%_{ddCa\left(OH\right)_2}=\dfrac{0,1.74.100\%}{500}=1,48\%\)
b) \(m_{Ca\left(OH\right)_2}=200.1,48=2,96\left(g\right)\Rightarrow n_{Ca\left(OH\right)_2}=\dfrac{2,96}{74}=0,04\left(mol\right)\)
PTHH: Ca(OH)2 + 2HCl → CaCl2 + 2H2O
Mol: 0,04 0,08
\(V_{ddHCl}=\dfrac{0,08}{2}=0,04\left(l\right)=40\left(ml\right)\)

\(Đặt.kim.loại.kiềm:A\\ 2A+2HCl\rightarrow2ACl+H_2\\ m_{muối}-m_{kl}=m_{Cl^-}\\ \Leftrightarrow m_{Cl^-}=7,45-3,9=3,55\left(g\right)\\ \Rightarrow n_{HCl}=n_{Cl^-}=\dfrac{3,55}{35,5}=0,1\left(mol\right)\\ \Rightarrow n_A=n_{ACl}=n_{HCl}=0,1\left(mol\right)\\ a,M_A=\dfrac{3,9}{0,1}=39\left(\dfrac{g}{mol}\right)\\ \Rightarrow A\left(I\right):Kali\left(K=39\right)\\ b,n_{H_2}=\dfrac{n_{HCl}}{2}=\dfrac{0,1}{2}=0,05\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\\ c,m_{ddHCl}=\dfrac{0,1.36,5.100}{31,7}=\dfrac{3650}{317}\left(g\right)\\ \Rightarrow V_{ddHCl}=\dfrac{\dfrac{3650}{317}}{1,15}\approx10,012\left(g\right)\)

a) nHCl = 0,1.1 = 0,1 (mol)
PTHH: 2R + 2H2O --> 2ROH + H2
0,1<---------------0,1---->0,05
ROH + HCl --> RCl + H2O
0,1<--0,1
=> \(M_R=\dfrac{2,3}{0,1}=23\left(g/mol\right)\)
=> R là Na
b) VH2 = 0,05.22,4 = 1,12(l)


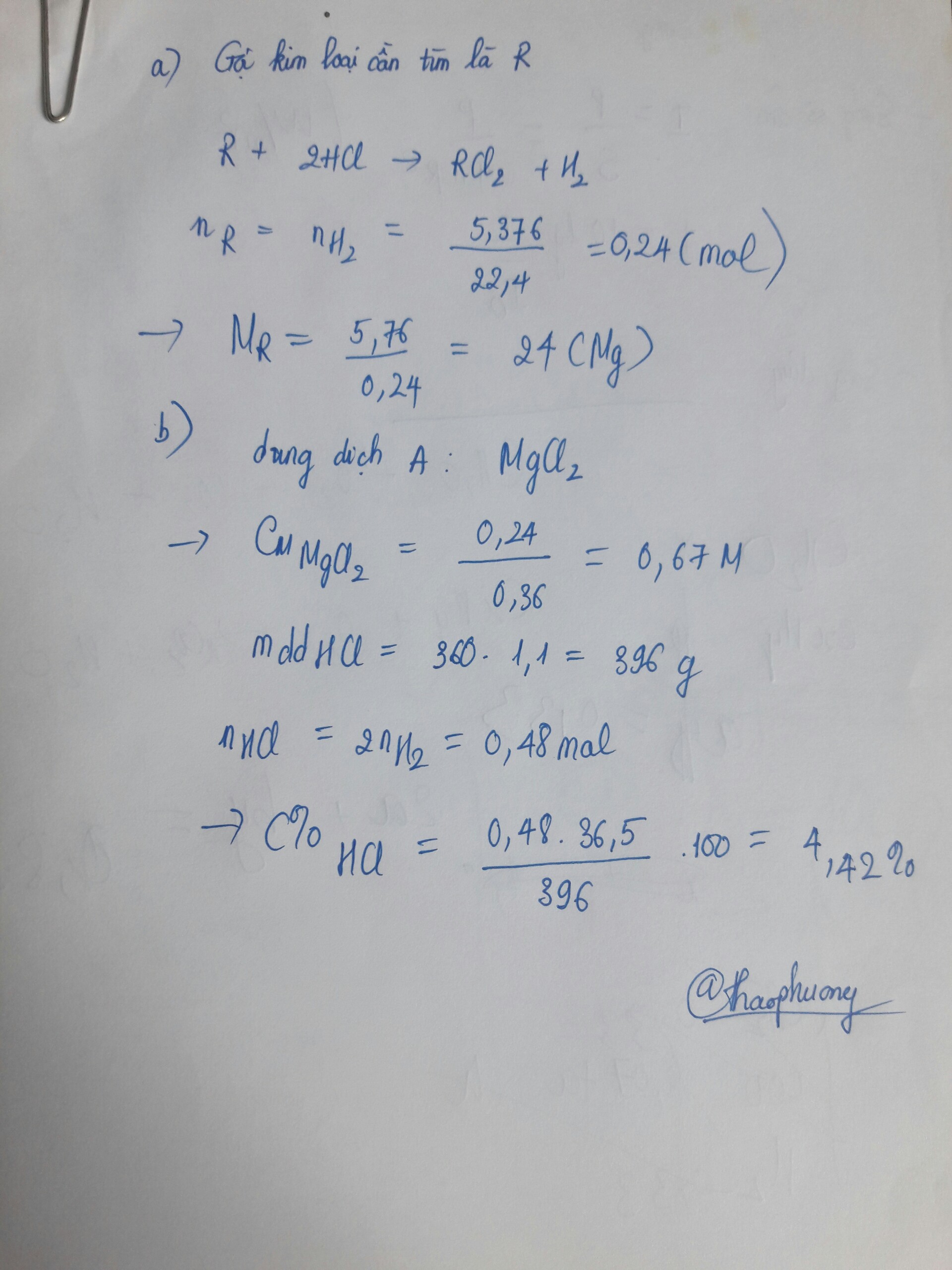
\(\text{Đ}\text{ặt}:A\\ A+HCl\rightarrow ACl+H_2\\ n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ n_A=n_{ACl_2}=2.n_{H_2}=0,1.2=0,2\left(mol\right)\\ M_{ACl}=\dfrac{11,7}{0,2}=58,5\left(\dfrac{g}{mol}\right)\\ M\text{à}:M_{ACl}=M_A+35,5\\ \Rightarrow M_A=23\left(\dfrac{g}{mol}\right)\\ \Rightarrow A:Natri\left(Na\right)\\ a=23.0,2=4,6\left(g\right)\)
nH2=2,24/22,4=0,1(mol)
2M+2HCl→2MCl+H2
0,2 ← 0,2 ← 0,1
Có 0,2 .(M+35,5)=11,7(gam)
⇒ M=23 ⇒M là Na
mNa=23. 0,2= 4,6 (gam)