Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Sửa đề : 3.36 (l)
\(n_{H_2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(0.1.........0.15...............0.05.......0.15\)
\(m_{Al}=0.1\cdot27=2.7\left(g\right)\)
\(m_{Al_2\left(SO_4\right)_3}=0.05\cdot342=17.1\left(g\right)\)
\(m_{H_2SO_4}=0.15\cdot98=14.7\left(g\right)\)

2Al+3H2SO4->Al2(SO4)3+3H2
0,1----------------------0,075----0,15
n H2=0,15 mol
=>mAl=0,1.27=2,7g
=>m Al2(SO4)3=0,075.342=25,65g
a) PTHH: \(2Al+3H_2SO_2\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
b) \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
\(n_{Al}=\dfrac{2}{3}.0,15=0,1\left(mol\right)\)
\(m_{Al}=0,1.27=2,7\left(g\right)\)
c) \(n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}.0,1=0,05\left(mol\right)\)
\(m_{Al_2\left(SO_4\right)_3}=0,05.342=17,1\left(g\right)\)

2Al+3H2SO4->Al2(SO4)3+3H2
0,2-----0,3-------0,1------------0,3
n Al=\(\dfrac{5,4}{27}\)=0,2 mol
n H2SO4= \(\dfrac{30}{98}\)=0,306 mol
=>H2SO4 còn dư
=>VH2=0,3.22,4=6,72l
=>m Al2(SO4)3=0,1.342=34,2g
=>m H2SO4 dư=0,006.98=0,588g
\(n_{Al}=\dfrac{m_{Al}}{M_{Al}}=\dfrac{5,4}{27}=0,2mol\)
\(n_{H_2SO_4}=\dfrac{m_{H_2SO_4}}{M_{H_2SO_4}}=\dfrac{30}{98}=\dfrac{15}{49}mol\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
2 3 1 3 ( mol )
0,2 15/49 ( mol )
Ta có: \(\dfrac{0,2}{2}< \dfrac{15}{49}:3\)
=> Chất còn dư là \(H_2SO_4\)
\(V_{H_2}=n_{H_2}.22,4=\left(\dfrac{0,2.3}{2}\right).22,4=6,72l\)
\(m_{Al_2\left(SO_4\right)_3}=n_{Al_2\left(SO_4\right)_3}.M_{Al_2\left(SO_4\right)_3}=\left(\dfrac{0,2.1}{2}\right).342=34,2g\)
\(m_{H_2SO_4\left(du\right)}=n_{H_2SO_4\left(du\right)}.M_{H_2SO_4}=\left(\dfrac{15}{49}-\dfrac{0,2.3}{2}\right).98=0,6g\)

a) Gọi số mol Fe2O3 là a (mol)
PTHH: Fe2O3 + 3H2 --to--> 2Fe + 3H2O
a---->3a--------->2a
=> 160a - 56.2a = 9,6
=> a = 0,2 (mol)
=> \(V_{H_2}=0,6.22,4=13,44\left(l\right)\)
b) m = 0,2.160 = 32 (g)
mFe = 0,4.56 = 22,4 (g)

a) Gọi số mol H2 phản ứng là a (mol)
PTHH: Fe2O3 + 3H2 --to--> 2Fe + 3H2O
mgiảm = mO(mất đi) = 4,8 (g)
=> nO(mất đi) = \(\dfrac{4,8}{16}=0,3\left(mol\right)\)
=> \(n_{H_2O}=0,3\left(mol\right)\)
=> \(n_{H_2}=0,3\left(mol\right)\)
=> \(V_{H_2}=0,3.22,4=6,72\left(l\right)\)
b) \(n_{Fe}=0,2\left(mol\right)\)
=> \(m_{Fe}=0,2.56=11,2\left(g\right)\)
\(n_{Fe_2O_3}=0,1\left(mol\right)\)
=> \(m_{Fe_2O_3}=0,1.160=16\left(g\right)\)

\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\)
\(nH_2=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
\(nH_2SO_4=nH_2=0,15\left(mol\right)\)
\(nH_2SO_{4\left(15\%\right)}=\dfrac{0,15.15}{100}=0,0225\left(mol\right)\)
\(mH_2SO_4=0,0225.98=2,205\left(g\right)\)
\(nAl=\dfrac{2}{3}.0,15=0,1\left(mol\right)\)
\(mAl=0,1.27=2,7\left(g\right)\)
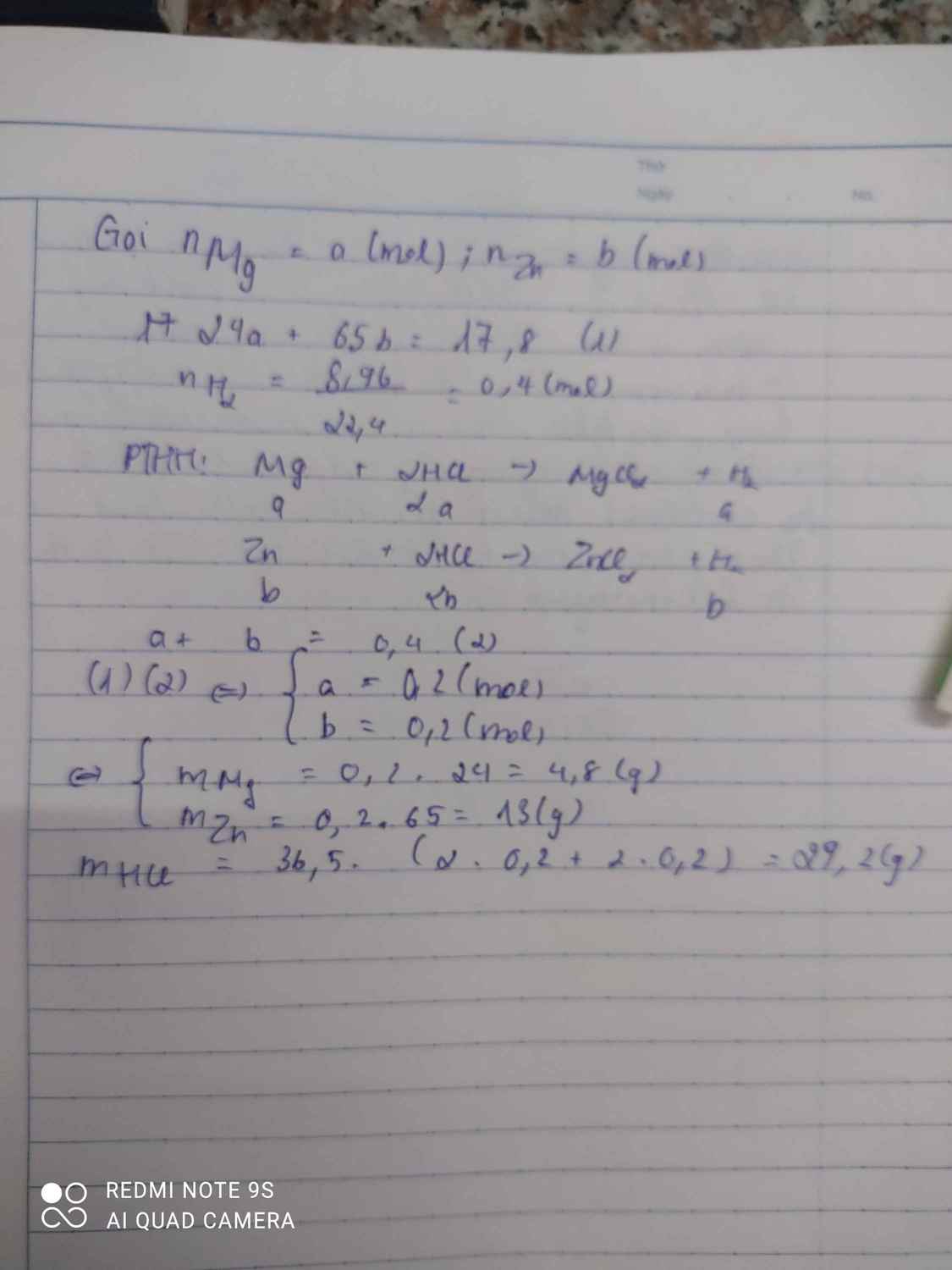
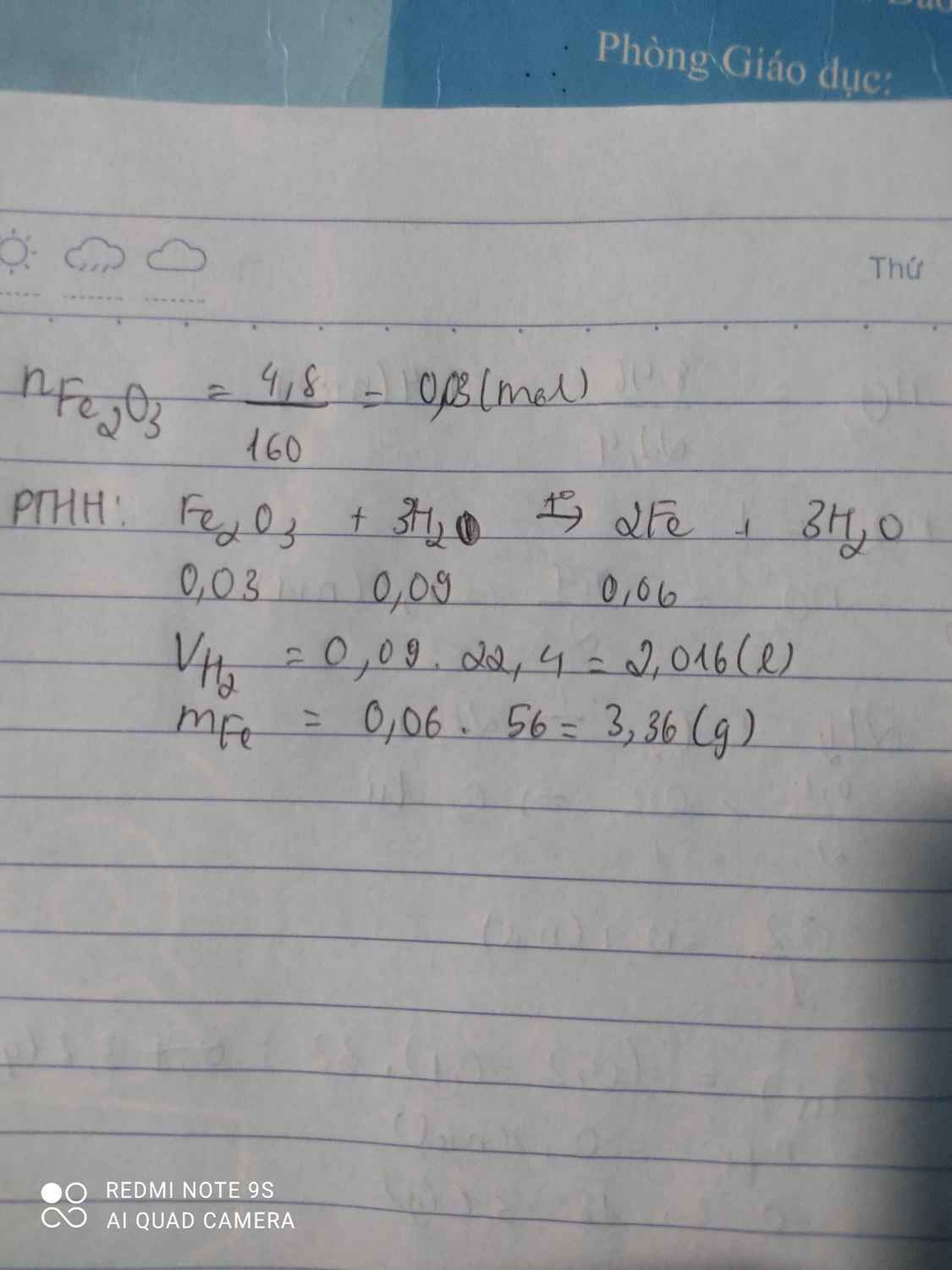
Giúp mik vs huhuh