Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,CH_3COOH+Na\rightarrow CH_3COONa+\dfrac{1}{2}H_2\\C_2H_5COOH+Na\rightarrow C_2H_5COONa+\dfrac{1}{2}H_2\\ n_{H_2}=0,0625\left(mol\right)\\ Đặt:n_{CH_3COOH}=a\left(mol\right);n_{C_2H_5COOH}=b\left(mol\right)\left(a,b>0\right)\\ \Rightarrow\left\{{}\begin{matrix}60a+74b=8,55\\0,5a+0,5b=0,0625\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,05\\b=0,075\left(mol\right)\end{matrix}\right.\\b,\%m_{CH_3COOH}=\dfrac{60.0,05}{8,55}.100\approx35,088\%\Rightarrow \%m_{C_2H_5COOH}\approx64,912\%\)

Số mol Br3C6H2OH = 19,86: 331,0 = 0,0600 (mol)
mphenol = 0,0600. 94,0 = 5,64 g
= 0,2400 mol
= 0,2400 . 46,0 = 11,05 g
Phần trăm khối lượng của etanol là 66,2% phần trăm khối lượng của phenol là 33,8%
lam sao ban ra dc nC2H5OH = 0,24 mol vay
ban ghiro ra di de cho tui hieu voi

`a)PTHH:`
`CH_3 OH+Na->CH_3 ONa+1/2H_2 \uparrow`
`C_2 H_5 OH+Na->C_2 H_5 ONa+1/2H_2 \uparrow`
`b)n_[H_2]=[0,448]/[22,4]=0,02(mol)`
Gọi `n_[CH_3 OH]=x;n_[C_2 H_5 OH]=y`
`=>` $\begin{cases} 32x+46y=1,56\\x+y=0,02.2 \end{cases}$
`<=>x=y=0,02`
`%m_[CH_3 OH]=[0,02.32]/[1,56].100=41,03%`
`%m_[C_2 H_5 OH]=100-41,03=58,97%`

a)
$Cu + 4HNO_3 \to Cu(NO_3)_2 + 2NO_2 + 2H_2O$
$Ag + 2HNO_3 \to AgNO_3 + NO_2 + H_2O$
b)
Gọi $n_{Cu} = a(mol) ; n_{Ag} = b(mol) \Rightarrow 64a + 108b = 4,52(1)$
$n_{NO_2} =2a + b = 0,07(2)$
Từ (1)(2) suy ra a = 0,02 ; b = 0,03
$\%m_{Cu} = \dfrac{0,02.64}{4,52}.100\% = 28,31\%$
$\%m_{Ag} = 71,69\%$

1. nKOH = 30/1000*2 = 0,06 mol
nCH3COOH = nKOH = 0,06 mol (bạn tự viết PTHH)
=> CCH3COOH=\(\frac{0,06}{0,05}=1,2M\)
=> Trong 125ml axit có chứa 0,06*125/50 = 0,15 mol CH3COOH
BTNT ta có nCH3COOH = nCH3COONa = 0,15 mol
=> m muối = m CH3COONa = 12,3 gam.
2. nH2= 0,5 mol
Gọi nCH3OH = a, nC6H5OH = b (mol)
PTTQ 2CH3-OH + 2Na → 2CH3-ONa + H2
2C6H5-OH + 2Na → 2C6H5-ONa + H2
=> nROH = nCH3OH + nC6H5OH = 2nH2
=> a + b = 1 mol
nC6H2Br3OH = 0,18 mol = nC6H5OH = b => a = 1-0,18 = 0,82 mol
=> mC6H5OH = 0,18*94 = 16,92g
mCH3OH = 26,24 gam
=> %C6H5OH = \(\frac{16,92\cdot100\%}{16,92+26,24}\)= 39,2%
=> %CH3OH = 60,8%

Giải thích: Đáp án: A
 => X chứa ACHO (a mol) và B(CHO)2 (b mol)
=> X chứa ACHO (a mol) và B(CHO)2 (b mol)
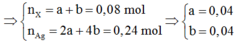
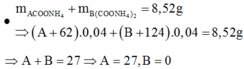
X chứa ![]()
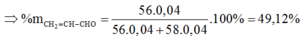
=> Chọn đáp án A.

a)
2C6H5OH + 2Na --> 2C6H5ONa + H2
2C2H5OH + 2Na --> 2C2H5ONa + H2
b)
Gọi số mol C6H5OH, C2H5OH là a, b (mol)
=> 94a + 46b = 5,12 (1)
\(n_{H_2}=\dfrac{0,896}{22,4}=0,04\left(mol\right)\)
PTHH: 2C6H5OH + 2Na --> 2C6H5ONa + H2
a---------------------------->0,5a
2C2H5OH + 2Na --> 2C2H5ONa + H2
b----------------------------->0,5b
=> 0,5a + 0,5b = 0,04 (2)
(1)(2) => a = 0,03 (mol); b = 0,05 (mol)
=> \(\left\{{}\begin{matrix}\%m_{C_6H_5OH}=\dfrac{0,03.94}{5,12}.100\%=55,08\%\\\%m_{C_2H_5OH}=\dfrac{0,05.46}{5,12}.100\%=44,92\%\end{matrix}\right.\)

a) nH2 = 0,125 mol
C2H5OH + Na → C2H5ONa + \(\dfrac{1}{2}\)H2
x.............................................\(\dfrac{x}{2}\)
C3H7OH + Na → C3H7ONa + \(\dfrac{1}{2}\)H2
y..............................................\(\dfrac{y}{2}\)
ta có \(\left\{{}\begin{matrix}46x+60y=12,2\\x+y=0,25\end{matrix}\right.\) \(\Leftrightarrow\) \(\left\{{}\begin{matrix}x=0,2\\y=0,05\end{matrix}\right.\)
→%C2H5OH = \(\dfrac{0,2.46}{12,2}.100\%\) \(\approx\) 75,41%
→%C3H7OH = 24,59%
b) phương trình
C2H5OH + CuO \(\underrightarrow{t^o}\) CH3COOH + Cu + H2O
C3H7OH + CuO \(\underrightarrow{t^o}\) C2H5COOH + Cu + H2O
\(a,HCOOH+Na\rightarrow HCOONa+\dfrac{1}{2}H_2\\ CH_3COOH+Na\rightarrow CH_3COONa+\dfrac{1}{2}H_2\\ n_{H_2}=0,075\left(mol\right)\\ Đặt:n_{HCOOH}=a\left(mol\right);n_{CH_3COOH}=b\left(mol\right)\left(a,b>0\right)\\ \Rightarrow\left\{{}\begin{matrix}46a+60b=7,95\\0,5a+0,5b=0,075\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,075\\b=0,075\end{matrix}\right.\\ b,\%m_{HCOOH}=\dfrac{0,075.46}{7,95}.100\%\approx43,396\%\Rightarrow\%m_{CH_3COOH}\approx56,604\%\)