Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\uparrow\)
a) Ta có: \(\left\{{}\begin{matrix}n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\n_{H_2SO_4}=\dfrac{200\cdot4,9\%}{98}=0,1\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) Cả 2 chất p/ứ hết
b+c) Theo PTHH: \(n_{ZnSO_4}=n_{H_2}=n_{Zn}=0,1\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{ZnSO_4}=0,1\cdot161=16,1\left(g\right)\\m_{H_2}=0,1\cdot2=0,2\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{Zn}+m_{ddH_2SO_4}-m_{H_2}=206,3\left(g\right)\)
\(\Rightarrow C\%_{ZnSO_4}=\dfrac{16,1}{206,3}\cdot100\%\approx7,8\%\)

\(n_{Zn}=\dfrac{6,5}{65}=0,1mol\)
\(m_{HCl}=\dfrac{200\cdot14,6\%}{100\%}=29,2g\Rightarrow n_{HCl}=0,8mol\)
a)\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,8 0 0
0,1 0,2 0,1 0,1
0 0,6 0,1 0,1
b)Chất HCl dư và dư \(m=0,6\cdot36,5=21,9g\)
c)\(V_{H_2}=0,1\cdot22,4=2,24l\)
d)\(m_{H_2}=0,1\cdot2=0,2g\)
\(m_{ZnCl_2}=0,1\cdot136=13,6g\)
\(m_{ddZnCl_2}=6,5+200-0,2=206,3g\)
\(C\%=\dfrac{13,6}{206,3}\cdot100\%=6,59\%\)
a, ta có pt sau : Zn + 2HCl >ZnCl2 + H2 (1)
b, nHCl=\(\dfrac{200\times14,6}{100}=29,2\left(g\right)\)\(\Rightarrow n_{HCl}=\dfrac{29,2}{36,5}=0,8\left(mol\right)\)
Ta có : nZn=\(\dfrac{6,5}{65}=0,1\left(mol\right)\)
Ta có tỉ lệ số mol là : \(\dfrac{n_{Zn}}{1}< \dfrac{n_{HCl}}{2}\left(\dfrac{0,1}{1}< \dfrac{0,8}{2}\right)\)
\(\Rightarrow\) HCl dư , Zn pứ hết
Theo pt : nHClpứ = 2.nZn=2.0,1=0,2(mol)
\(\Rightarrow\)nHCl dư = nHCl bđ - nHCl pứ = 0,8 - 0,2 = 0,6 (mol)
\(\Rightarrow\)mHCl dư=0,6.36,6=21,9 (g)
c,theo pt :nH2=nZn=0,1(mol)
\(\Rightarrow\)VH2=0,1.22,4=2,24(l)
d,Các chất có trong dung dịch sau pứ là: ZnCl2 , HCl dư
mk chịu câu này ![]()

\(n_{Zn}=\dfrac{6.5}{65}=0.1\left(mol\right)\)
\(n_{HCl}=\dfrac{100\cdot14.6\%}{36.5}=0.4\left(mol\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(1........2\)
\(0.1......0.4\)
\(LTL:\dfrac{0.1}{1}< \dfrac{0.4}{2}\Rightarrow HCldư\)
\(V_{H_2}=0.1\cdot22.4=2.24\left(l\right)\)
\(m_{\text{dung dịch sau phản ứng}}=6.5+100-0.1\cdot2=106.3\left(g\right)\)
\(C\%ZnCl_2=\dfrac{0.1\cdot136}{106.3}\cdot100\%=12.79\%\)
\(C\%HCl\left(dư\right)=\dfrac{\left(0.4-0.2\right)\cdot36.5}{106.3}\cdot100\%=6.87\%\%\)

\(a,n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\\ m_{H_2SO_4}=200.19,6\%=39,2\left(g\right)\\ \rightarrow n_{H_2SO_4}=\dfrac{39,2}{98}=0,4\left(mol\right)\)
PTHH: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\uparrow\)
bđ 0,3 0,4
pư 0,3 0,3
spư 0 0,1 0,3 0,3
\(\rightarrow V_{H_2}=0,3.22,4=6,72\left(l\right)\)
\(b,m_{dd}=19,5+200-0,3.2=218,9\left(g\right)\\ \rightarrow\left\{{}\begin{matrix}C\%_{ZnSO_4}=\dfrac{0,3.161}{218,9}.100\%=22,06\%\\C\%_{H_2SO_4\left(dư\right)}=\dfrac{0,1.98}{218,9}.100\%=4,48\%\end{matrix}\right.\)

a) \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
\(n_{H_2SO_4}=1.0,2=0,2\left(mol\right)\)
PTHH: Zn + H2SO4 --> ZnSO4 + H2
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,2}{1}\) => Zn hết, H2SO4 dư
b)
PTHH: Zn + H2SO4 --> ZnSO4 + H2
0,1--->0,1------->0,1
=> \(m_{ZnSO_4}=0,1.161=16,1\left(g\right)\)
c) \(\left\{{}\begin{matrix}C_{M\left(H_2SO_4.dư\right)}=\dfrac{0,2-0,1}{0,2}=0,5M\\C_{M\left(ZnSO_4\right)}=\dfrac{0,1}{0,2}=0,5M\end{matrix}\right.\)

a, \(n_{HNO_3}=0,3.1=0,3\left(mol\right)\)
\(n_{Ba\left(OH\right)_2}=0,1.1=0,1\left(mol\right)\)
PT: \(2HNO_3+Ba\left(OH\right)_2\rightarrow Ba\left(NO_3\right)_2+2H_2O\)
Xét tỉ lệ: \(\dfrac{0,3}{2}>\dfrac{0,1}{1}\), ta được HNO3 dư.
Theo PT: \(\left\{{}\begin{matrix}n_{Ba\left(NO_3\right)_2}=n_{Ba\left(OH\right)_2}=0,1\left(mol\right)\\n_{HNO_3\left(pư\right)}=2n_{Ba\left(OH\right)_2}=0,2\left(mol\right)\end{matrix}\right.\)
⇒ nHNO3 (dư) = 0,3 - 0,2 = 0,1 (mol)
\(\Rightarrow\left\{{}\begin{matrix}C_{M_{Ba\left(NO_3\right)_2}}=\dfrac{0,1}{0,3+0,1}=0,25\left(M\right)\\C_{M_{HNO_3\left(dư\right)}}=\dfrac{0,1}{0,3+0,1}=0,25\left(M\right)\end{matrix}\right.\)
b, Ta có: \(n_{Na_2CO_3}=0,25.0,5=0,125\left(mol\right)\)
PT: \(Na_2CO_3+2HNO_3\rightarrow2NaNO_3+CO_2+H_2O\)
______0,05______0,1_______________0,05 (mol)
⇒ VCO2 = 0,05.22,4 = 1,12 (l)
\(Na_2CO_3+Ba\left(NO_3\right)_2\rightarrow2NaNO_3+BaCO_{3\downarrow}\)
0,075________0,075_______________0,075 (mol)
⇒ mBaCO3 = 0,075.197 = 14,775 (g)

nFe = 5.6/56 = 0.1 (mol)
nHCl = 0.2*2 = 0.4 (mol)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
LTL : 0.1/1 < 0.4/2 => HCl dư
mHCl dư = ( 0.4 - 0.2 ) * 36.5 = 7.3 (g)
VH2 = 0.2*22.4 = 4.48 (l)
CM FeCl2 = 0.1/0.2 = 0.5(M)
CM HCl dư = 0.2 / 0.2 = 1(M)

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\\ m_{HCl}=\dfrac{109,5\cdot10\%}{100\%}=10,95\left(g\right)\\ \Rightarrow n_{HCl}=\dfrac{10,95}{36,5}=0,3\left(mol\right)\\ a,PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\\ \text{Vì }\dfrac{n_{Mg}}{1}< \dfrac{n_{HCl}}{2}\text{ nên sau p/ứ }HCl\text{ dư}\\ \Rightarrow n_{H_2}=0,1\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=0,1\cdot22,4=2,24\left(l\right)\)
\(b,n_{MgCl_2}=0,1\left(mol\right)\\ \Rightarrow m_{CT_{MgCl_2}}=0,1\cdot95=9,5\left(g\right)\\ m_{H_2}=0,1\cdot2=0,2\left(mol\right)\\ m_{dd_{MgCl_2}}=2,4+109,5-0,2=111,7\left(g\right)\\ \Rightarrow C\%_{MgCl_2}=\dfrac{9,5}{111,7}\cdot100\%\approx8,5\%\)


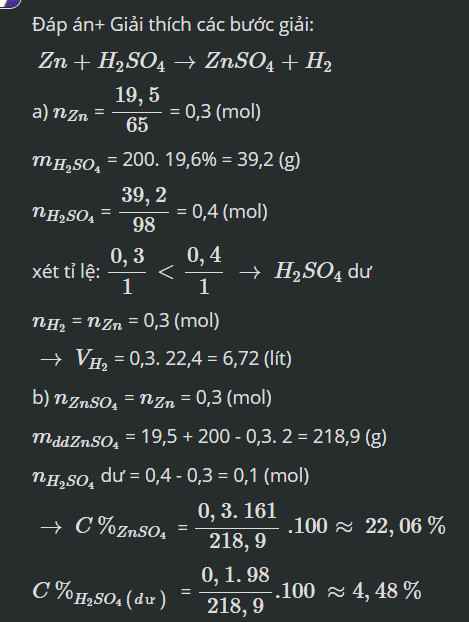
\(n_{Zn}=\dfrac{6,5}{65}=0,1mol\)
\(m_{FeSO_4}=\dfrac{200\cdot15,2}{100}=30,4g\Rightarrow n_{FeSO_4}=0,2mol\)
\(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
0,1 0,2
\(\Rightarrow\) Zn hết, H2SO4 dư 0,1mol.
a, Có: \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
\(m_{FeSO_4}=200.15,2\%=30,4\left(g\right)\Rightarrow n_{FeSO_4}=\dfrac{30,4}{152}=0,2\left(mol\right)\)
PT: \(Zn+FeSO_4\rightarrow ZnSO_4+Fe\)
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,2}{1}\), ta được FeSO4 dư, Zn hết.
b, Theo PT: \(n_{ZnSO_4}=n_{Fe}=n_{FeSO_4Z\left(pư\right)}=n_{Zn}=0,1\left(mol\right)\)
\(\Rightarrow n_{FeSO_4\left(dư\right)}=0,1\left(mol\right)\)
Có: m dd sau pư = 6,5 + 200 - 0,1.56 = 200,9 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{ZnSO_4}=\dfrac{0,1.161}{200,9}.100\%\approx8,01\%\\C\%_{FeSO_4\left(dư\right)}=\dfrac{0,1.152}{200,9}.100\%\approx7,57\%\end{matrix}\right.\)
Bạn tham khảo nhé!