Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Đáp án D
Xem hỗn hợp X gồm M2O, M2O2, M2CO3
Hỗn hợp Y gồm MCl, HCl.


BT e: \(3n_{Al}+2n_{Mg}=4n_{O_2}+2n_{Cl_2}\)
\(\Rightarrow3\cdot0,1+2\cdot0,05=4\cdot0,05+2\cdot x\)
\(\Rightarrow x=0,1mol\)
\(m=m_{Al}+m_{Mg}+m_{O_2}+m_{Cl_2}\)
\(=0,1\cdot27+0,05\cdot24+0,05\cdot2\cdot16+0,1\cdot35,5\cdot2\)
\(=12,6g\)
Theo bảo toàn electron ta có: \(3\cdot n_{Al}+2\cdot n_{Mg}=2\cdot n_{Cl_2}+4\cdot N_{O_2}\)
\(\Rightarrow3\cdot0,1+2\cdot0,05=4\cdot0,05+2x\Rightarrow x=0,2\)
\(\Rightarrow m_Z=m_X+m_Y=0,1\cdot27+0,05\cdot24+0,05\cdot32+0,2\cdot71=19,7g\)

PTHH: Fe + 2HCl --> FeCl2 + H2
FeS + 2HCl --> FeCl2 + H2S
=> \(n_{Fe}+n_{FeS}=n_{H_2}+n_{H_2S}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
Và 56.nFe + 88.nFeS = 18,8
=> \(\left\{{}\begin{matrix}n_{Fe}=0,1\left(mol\right)\\n_{FeS}=0,15\left(mol\right)\end{matrix}\right.\)
Bảo toàn S: nCaSO3 = 0,15 (mol)
=> m = 0,15.120 = 18 (g)
=> B

Chọn đáp án D
n H C l = 2 n O o x i t = 2 3 , 43 - 2 , 15 16 =0,16 (mol)
=> V d d H C l = 0 , 16 0 , 5 = 0,32 (lít)
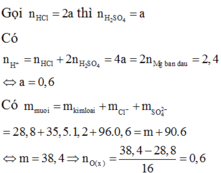
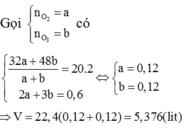
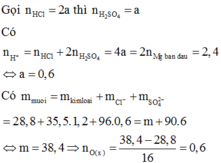
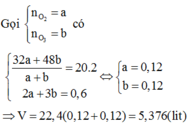
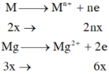
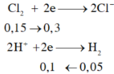
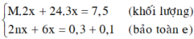
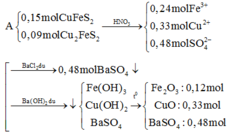
Gọi \(\left\{{}\begin{matrix}n_{O2}:a\left(mol\right)\\n_{O3}:b\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}a+b=0,2\\\frac{32a+48b}{0,2}=20.2=40\end{matrix}\right.\)\(\Rightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\)
\(\left\{{}\begin{matrix}n_C:x\left(mol\right)\\n_S:y\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}12x+32y=5,6\\4x+4y=0,1.4+0,1.6=1\left(BT.e\right)\end{matrix}\right.\)\(\Rightarrow\left\{{}\begin{matrix}a=0,12\\b=0,13\end{matrix}\right.\)
\(\Rightarrow\frac{n_C}{n_S}=\frac{12}{13}\)
Đặt 12a, 13b là mol C,S trong 3,36g X
\(\Rightarrow12.12a+32.13a=3,36\)
\(\Rightarrow a=0,006\)
\(\left\{{}\begin{matrix}n_C=0,072\left(mol\right)\\n_S=0,078\left(mol\right)\end{matrix}\right.\underrightarrow{^{t^o}}\left\{{}\begin{matrix}CO_2=0,072\left(mol\right)\\SO_2=0,078\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\Sigma n_{khi}=0,15\left(mol\right)\)
\(n_{OH}=n_{NaOH}+n_{KOH}=0,2\left(mol\right)\)
\(\frac{n_{OH}}{n_{khi}}=1,3\Rightarrow\) Tạo 2 loại muối
Gọi chung CO2, SO2 là XO2 :\(\left\{{}\begin{matrix}n_{XO2}=0,15\left(mol\right)\\m_{XO2}=8,16\left(g\right)\end{matrix}\right.\)
\(\Rightarrow\overline{M_{XO2}}=\frac{8,16}{0,15}=54,4\)
\(\Rightarrow\overline{M_X}=22,4\)
Gọi chung NaOH ,KOH là ROH : \(\left\{{}\begin{matrix}n_{ROH}=0,25\left(mol\right)\\n_{ROH}=8,8\left(g\right)\end{matrix}\right.\)
\(\Rightarrow\overline{M_{ROH}}=\frac{8,8}{0,25}=35,2\)
\(\Rightarrow\overline{M_R}=18,2\)
\(ROH+XO_2\rightarrow RHXO_3\)
a________a_________a
\(2ROH+XO_2\rightarrow R_2XO_3+H_2O\)
2b________b_____b____________
\(\Rightarrow\left\{{}\begin{matrix}a+2b=0,25\\a+b=0,15\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}a=0,05\\b=0,1\end{matrix}\right.\)
\(\Rightarrow m=m_{RHXO3}+m_{R2XO3}=15,16\left(g\right)\)