Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

nMg = 0,1(mol)
PTHH: Mg + 2HCl --> MgCl2 +H2
nMg = nMgCl2= nH2 = 0,1(mol)
=> mmuối = 9,5(g)
VH2 = 2,24(l)
b) CMHCl = 0,2/0,1=2(M)

Gọi \(\left\{{}\begin{matrix}n_{Zn}=a\left(mol\right)\\n_{Fe}=b\left(mol\right)\\n_{Al}=c\left(mol\right)\end{matrix}\right.\) => 65a + 56b + 27c = 10,65 (1)
PTHH: Zn + 2HCl --> ZnCl2 + H2
Fe + 2HCl --> FeCl2 + H2
2Al + 6HCl --> 2AlCl3 + 3H2
=> \(n_{H_2}=a+b+1,5c=\dfrac{5,04}{22,4}=0,225\left(mol\right)\) (2)
PTHH: Zn + Cl2 --to--> ZnCl2
2Fe + 3Cl2 --to--> 2FeCl3
2Al + 3Cl2 --to--> 2AlCl3
=> \(n_{Cl_2}=a+1,5b+1,5c=\dfrac{5,6}{22,4}=0,25\left(mol\right)\) (3)
(1)(2)(3) => \(\left\{{}\begin{matrix}a=0,1\left(mol\right)\\b=0,05\left(mol\right)\\c=0,05\left(mol\right)\end{matrix}\right.\) => \(\left\{{}\begin{matrix}m_{Zn}=0,1.65=6,5\left(g\right)\\m_{Fe}=0,05.56=2,8\left(g\right)\\m_{Al}=0,05.27=1,35\left(g\right)\end{matrix}\right.\)
a) \(\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{6,5}{10,65}.100\%=61,033\%\\\%m_{Fe}=\dfrac{2,8}{10,65}.100\%=26,291\%\\\%m_{Al}=\dfrac{1,35}{10,65}.100\%=12,676\%\end{matrix}\right.\)
b) nHCl = 2a + 2b + 3c = 0,45 (mol)
=> mHCl = 0,45.36,5 = 16,425 (g)
=> \(a\%=C\%=\dfrac{16,425}{200}.100\%=8,2125\%\)
c) mdd sau pư = 10,65 + 200 - 0,225.2 = 210,2 (g)
=> \(\left\{{}\begin{matrix}C\%_{ZnCl_2}=\dfrac{0,1.136}{210,2}.100\%=6,47\%\\C\%_{FeCl_2}=\dfrac{0,05.127}{210,2}.100\%=3,02\%\\C\%_{AlCl_3}=\dfrac{0,05.133,5}{210,2}.100\%=3,176\%\end{matrix}\right.\)

\(n_{HX}=\dfrac{1,568}{22,4}=0,07\left(mol\right)\\ X_2+H_2\rightarrow\left(tu\text{ỳ}.\text{Đ}K\right)2HX\\ n_{X_2}=\dfrac{0,07}{2}=0,035\left(mol\right)\\ M_{X_2}=\dfrac{2,485}{0,035}=71\left(\dfrac{g}{mol}\right)\\ \Rightarrow M_X=35,5\left(\dfrac{g}{mol}\right)\\ \Rightarrow X:Clo\left(Cl=35,5\right)\)

Câu 1:
Gọi đơn chất halogen là X2
\(Cu+X_2\underrightarrow{^{to}}CuX_2\)
Ta có:
\(n_X=\frac{5,6}{22,4}=0,25\left(mol\right)=n_{CuX2}\)
\(\Rightarrow M_{CuX2}=64+2X=\frac{33,75}{0,25}=135\)
\(\Rightarrow X=35,5\left(Clo\right)\)
Halogen là Cl2 - Clo
Câu 2:
\(PTHH:Fe+2HCl\rightarrow FeCl_2+H_2\)
_______0,15__0,3___0,15___0,15__
\(n_{Fe}=\frac{8,4}{56}=0,15\left(mol\right)\)
a,\(V_{H2}=0,15.22,4=3,36\left(l\right)\)
b, \(CM_{HCl}=\frac{0,3}{0,2}=1,5M\)

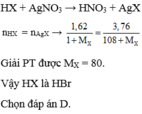
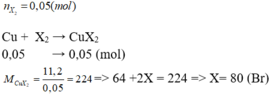
\(m_{HX}=\dfrac{10,95.200}{100}=21,9\left(g\right)\)
\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
PTHH: 2Al + 6HX --> 2AlX3 + 3H2
0,2--->0,6-------------->0,3
=> \(M_{HX}=\dfrac{21,9}{0,6}=36,5\left(g/mol\right)\)
=> X là Cl
VH2 = 0,3.22,4 = 6,72(l)