Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Phần trăm về khối lượng của CH3CHO là 44,0%, của CH3COOH LÀ 56,0%. Thể tích dung dịch NaOH là 0,46 lít.

\(a)n_{Ag} = \dfrac{21,6}{108} = 0,2(mol)\\ CH_3CHO + 2AgNO_3 + 3NH_3 + H_2O \to CH_3COONH_4 + 2Ag + 2NH_4NO_3\\ n_{CH_3CHO} = \dfrac{1}{2}n_{Ag} = 0,1(mol)\\ \Rightarrow C\%_{CH_3CHO} = \dfrac{0,1.44}{50}.100\% = 8,8\%\\ b) CH_3CHO + H_2 \xrightarrow{t^o,xt} CH_3CH_2OH\\ n_{CH_3CH_2OH} = n_{CH_3CHO} = 0,1(mol)\\ \Rightarrow m_{CH_3CH_2OH} = 0,1.46 = 4,6(gam)\)
a)nAg = 0,2 mol
CH3CHO + 2AgNO3 + 3NH3 + H2O → CH3COONH4 + 2NH4NO3 + 2Ag
0,1.......................................................................................................0,2
→mCH3CHO = 0,1. 44 = 4,4 g
→%CH3CHO = \(\dfrac{4,4}{5}\) .100% = 88%
b) CH3CHO + H2 → C2H5OH

Câu 19:
\(n_{Ag}=\dfrac{12,96}{108}=0,12\left(mol\right)\)
PT: \(CH_3CHO+2AgNO_3+3NH_3\underrightarrow{t^o}CH_3COONH_4+2Ag+2NH_4NO_3\)
Theo PT: \(n_{CH_3CHO}=\dfrac{1}{2}n_{Ag}=0,06\left(mol\right)\)
\(\Rightarrow C\%_{CH_3CHO}=\dfrac{0,06.44}{32}.100\%=8,25\%\)
Đáp án: C

CH3CHO + 2AgNO3 + 3NH3 + H2O → CH3COONH4 + 2Ag↓ + 2NH4NO3 (1)
CH3COOH + NaOH → CH3COONa + H2O (2)
Theo (1):
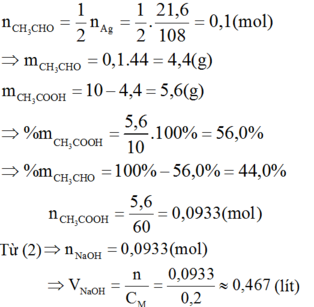

CH3CHO + 2AgNO3 + 3NH3 + H2O → CH3COONH4 + 2Ag↓ + 2NH4NO3 (1)
CH3COOH + NaOH → CH3COONa + H2O (2)

a) C2H4 + O2 -> CH3CHO
Hỗn hợp khí X gồm C2H4 chưa phản ứng và CH3CHO. Khi X tác dụng với dung dịch AgNO3/NH3
CH3CHO + 2AgNO3 + 3NH3 + H2O -> CH3COONH4 + 2Ag + 2NH4NO3
Số mol Ag = 0,150 mol. Vậy số mol CH3CHO = 0,0750 mol
Hiệu suất của quá trình oxi hóa etilen :  .100% = 75%
.100% = 75%
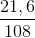 = 0,2 (mol)
= 0,2 (mol)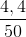 . 100% = 8,8%
. 100% = 8,8%
CH3CHO + 2AgNO3 + 3NH3 + H2O → CH3COONH4 + 2Ag↓ + 2NH4NO3
Từ phương trình ta có: