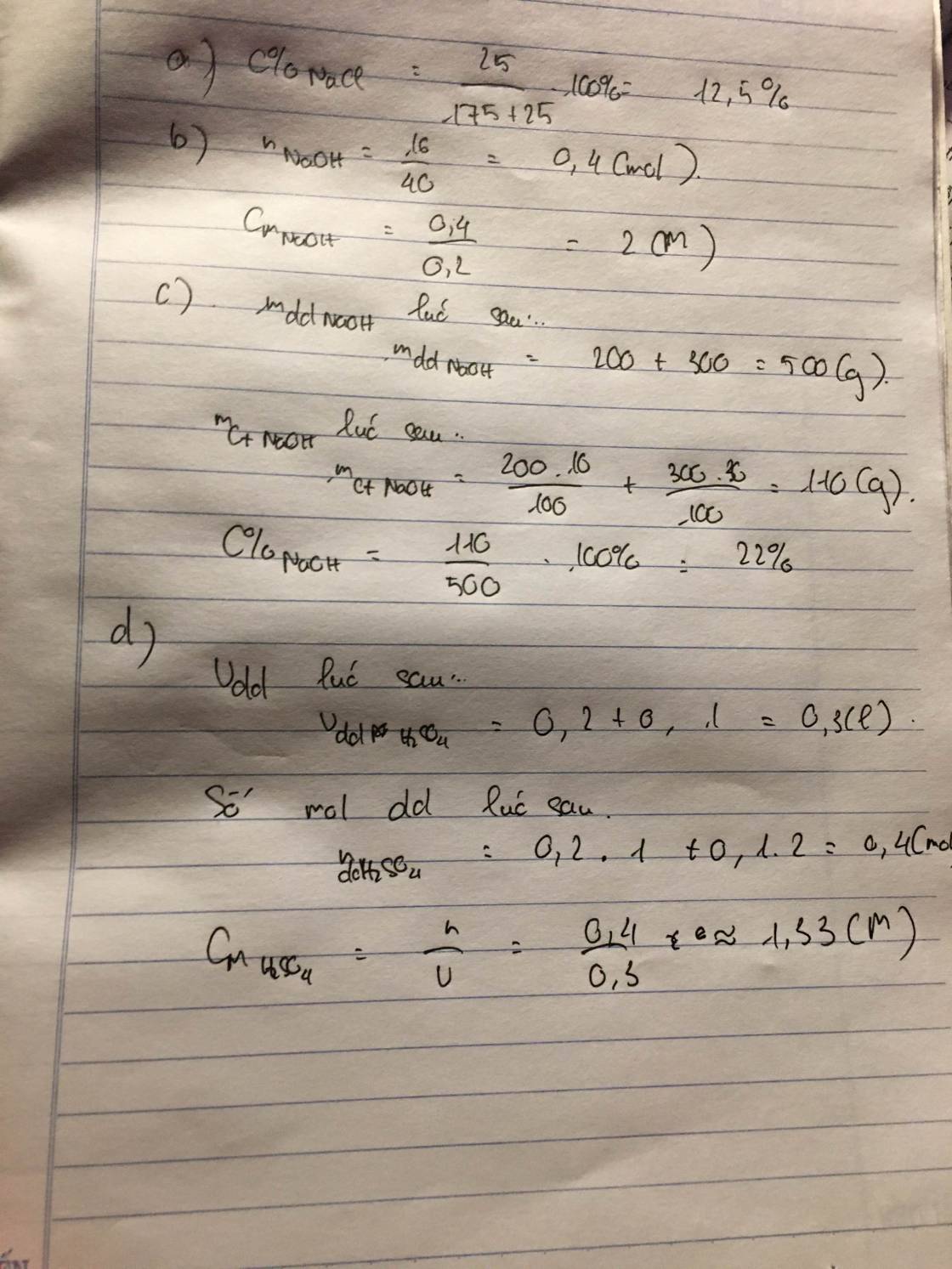Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(n_{Na_2O}=\dfrac{12,4}{62}=0,2\left(mol\right)\)
PTHH: Na2O + H2O ---> 2NaOH
0,2------------------>0,4
\(\Rightarrow C\%_{NaOH}=\dfrac{0,4.40}{12,4+50}.100\%=25,64\%\)
b, \(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\)
PTHH: 2Na + 2H2O ---> 2NaOH + H2
0,2------------------->0,2------->0,1
\(\Rightarrow C\%_{NaOH}=\dfrac{0,2.40+16}{100+16+4,6-0,1.2}.100\%==20\%\)
c, \(n_{Na}=\dfrac{9,2}{23}=0,4\left(mol\right)\)
\(n_{HCl}=\dfrac{100.7,3\%}{36,5}=0,2\left(mol\right)\)
PTHH:
2Na + 2HCl ---> 2NaCl + H2
0,2<-----0,2-----------0,2--->0,1
2Na + 2H2O ---> 2NaOH + H2
0,2------------------>0,2----->0,1
\(\Rightarrow m_{dd}=9,2+100-\left(0,1+0,1\right).2=108,8\left(g\right)\\ \Rightarrow\left\{{}\begin{matrix}C\%_{NaCl}=\dfrac{0,2.58,5}{108,8}.100\%=10,75\%\\C\%_{NaOH}=\dfrac{0,2.40}{108,8}.100\%=7,35\%\end{matrix}\right.\)

Hai bạn làm sai một số chỗ, mình sẽ làm lại
Bài 1:
\(Na_2O\left(0,1\right)+H_2O--->2NaOH\left(0,2\right)\)
\(n_{Na_2O}=0,1\left(mol\right)\)
\(\Rightarrow m_{NaOH}=0,2.40=8\left(g\right)\)
\(m_{ddsau}=6,2+73,8=80\left(g\right)\)
\(\Rightarrow C\%_{NaOH}=\dfrac{8}{80}.100=10\%\)
Bài 2:
\(n_{Na_2O}=0,1\left(mol\right)\)
\(m_{NaOH}\left(bđ\right)=60\left(g\right)\)
\(\Rightarrow m_{H_2O}=133,8-60=73,8\left(g\right)\)\(\Rightarrow n_{H_2O}=4,1\left(mol\right)\)
\(Na_2O\left(0,1\right)+H_2O\left(0,1\right)--->2NaOH\left(0,2\right)\)
So sánh: \(\dfrac{n_{Na_2O}}{1}=0,1< \dfrac{n_{H_2O}}{1}=4,1\)
=> Chọn số mol của Na2O để tính
Theo PTHH: nNaOH (tạo thành) = 0,2 (mol)
=> mNaOH (tạo thành) = 8 (g)
\(\Rightarrow\sum m_{NaOH}\left(sau\right)=60+8=68\left(g\right)\)
\(m_{ddsau}=6,2+133,8=140\left(g\right)\)
\(\Rightarrow C\%_{NaOH}\left(sau\right)=\dfrac{68}{140}.100=48,57\%\)
Bài 3:
\(m_{NaOH}\left(bđ\right)=12\left(g\right)\)
\(n_{Na_2O}=\dfrac{a}{62}\left(mol\right)\)
\(Na_2O\left(\dfrac{a}{62}\right)+H_2O--->2NaOH\left(\dfrac{a}{31}\right)\)
\(m_{NaOH}\left(tao.thanh\right)=\dfrac{a}{31}.40=\dfrac{40a}{31}\left(g\right)\)
\(\Rightarrow\sum m_{NAoh}\left(sau\right)=12+\dfrac{40a}{31}\left(g\right)\)
\(m_{ddsau}=\left(a+120\right)\left(g\right)\)
Ta có: \(20=\dfrac{12+\dfrac{40a}{31}}{a+120}.100\)
\(\Rightarrow a=11\left(g\right)\)
BT 1:
mdd = mct + mdm = 6,2 + 73,8 = 80 (g)
C%A = \(\dfrac{m_{ct}}{m_{dd}}.100=\dfrac{6,2}{80}.100=7,75\%\)

Cho 4,6 gam Na vào 100 gam H2O
1) Tính thể tích của H2
2) Tính nồng độ của dung dịch thu được
3) Tính Cm của dung dịch thu được
nNa=4,6/23=0,2(mol)
nH2O = 100/18=5,56(mol)
2Na + 2H2O---> 2NaOH + H2
0,2...<..5,56............0,2.............0,1
VH2=0,1.22,4=2,24(l)
C% NaOH = \(\frac{0,2.40}{4,6+100-0,1.2}.100\%=7,66\%\)

a, \(Na_2O+H_2O\rightarrow2NaOH\)
Ta có: \(n_{Na_2O}=\dfrac{3,1}{62}=0,05\left(mol\right)\)
Theo PT: \(n_{NaOH}=2n_{Na_2O}=0,1\left(mol\right)\)
m dd sau pư = 3,1 + 50 = 53,1 (g)
\(\Rightarrow C\%_{NaOH}=\dfrac{0,1.40}{53,1}.100\%\approx7,53\%\)
b, \(2Na+2H_2O\rightarrow2NaOH+H_2\)
Ta có: \(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\)
Theo PT: \(\left\{{}\begin{matrix}n_{NaOH}=n_{Na}=0,2\left(mol\right)\\n_{H_2}=\dfrac{1}{2}n_{Na}=0,1\left(mol\right)\end{matrix}\right.\)
Ta có: m dd sau pư = 4,6 + 95,6 - 0,1.2 = 100 (g)
\(\Rightarrow C\%_{NaOH}=\dfrac{0,2.40}{100}.100\%=8\%\)

a, Gọi \(m_{NaCl\left(thêm\right)}=a\left(g\right)\)
\(m_{NaCl\left(bđ\right)}=5\%.100=5\left(g\right)\\ \Rightarrow C\%_{NaCl}=\dfrac{5+a}{100+a}.100\%=5,5\%\\ \Leftrightarrow a=0,53\left(g\right)\)
b, \(m_{NaCl}=58,5.5,5\%=3,2175\left(g\right)\\ n_{NaCl}=\dfrac{3,2175}{58,5}=0,055\left(mol\right)\)
PTHH: NaCl + AgNO3 ---> AgCl↓ + NaNO3
0,055-->0,055------>0,055---->0,055
\(m_{AgCl}=0,055.143,5=7,8925\left(g\right)\\ m_{ddY}=58,5+200-7,8925=250,6075\left(g\right)\\ \Rightarrow C\%_{NaNO_3}=\dfrac{0,055.85}{250,6075}.100\%=1,87\%\)

\(Na+H_2O->NaOH+\dfrac{1}{2}H_2\\ a.n_{Na}=\dfrac{m_1}{23}\left(mol\right)\\ m_{ddsau}=\dfrac{m_1}{23}+m_2-\dfrac{m_1}{46}=\dfrac{m_1}{46}+m_2\left(g\right)\\ C\%_B=\dfrac{\dfrac{40}{23}m_1}{\dfrac{m_1}{46}+m_2}\cdot100\%.\\ b.C_M=\dfrac{10dC\%}{M}=10\cdot1,2\cdot\dfrac{0,05}{40}=0,015\left(M\right)\)
\(Na+H_2O->NaOH+\dfrac{1}{2}H_2\\ a.n_{Na}=\dfrac{m_1}{23}\left(mol\right)\\ m_{ddsau}=m_1+m_2-\dfrac{m_1}{23}=\dfrac{22}{23}m_1+m_2\left(g\right)\\ C\%_B=\dfrac{\dfrac{40}{23}m_1}{\dfrac{22}{23}m_1+m_2}\cdot100\%.\\ b.C_M=\dfrac{10dC\%}{M}=10\cdot1,2\cdot\dfrac{0,05}{40}=0,015\left(M\right)\)

\(n_{KOH}=\dfrac{400.7\%}{56}=0,5\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{100.19,6\%}{98}=0,2\left(mol\right)\)
PTHH: 2KOH + H2SO4 --> K2SO4 + 2H2O
Xét tỉ lệ: \(\dfrac{0,5}{2}>\dfrac{0,2}{1}\) => KOH dư, H2SO4 hết
PTHH: 2KOH + H2SO4 --> K2SO4 + 2H2O
0,4<----0,2-------->0,2
=> \(\left\{{}\begin{matrix}m_{KOH\left(dư\right)}=\left(0,5-0,4\right).56=5,6\left(g\right)\\m_{K_2SO_4}=0,2.174=34,8\left(g\right)\end{matrix}\right.\)
mdd sau pư = 400 + 100 = 500 (g)
=> \(\left\{{}\begin{matrix}C\%_{KOH.dư}=\dfrac{5,6}{500}.100\%=1,12\%\\C\%_{K_2SO_4}=\dfrac{34,8}{500}.100\%=6,96\%\end{matrix}\right.\)

\(n_{KOH}=\dfrac{400.7}{100}:56=0,5\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{100.19,6}{100}:98=0,2\left(mol\right)\)
\(2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O\)
0,4 0,2 0,2
Lập tỉ lệ:
\(\dfrac{0,5}{2}>\dfrac{0,2}{1}\) => KOH dư.
\(m_{dd}=400+100=500\left(g\right)\)
\(n_{KOH.dư}=0,5-0,4=0,1\left(mol\right)\)
\(C\%_{K_2SO_4}=\dfrac{0,2.174.100}{500}=6,96\%\)
\(C\%_{KOH}=\dfrac{0,1.56.100}{500}=1,12\%\)

nNa = 6.9 : 23 = 0.3 mol
4Na + O2 ->2 Na2O
mol : 0.3 -> 0.15
Na2O + H2O -> 2NaOH
mol : 0.15 -> 0.3
mdd = 0.15 x 62 + 140.7 = 150g
C% NaOH = 0.3x40: 150 x 100% = 8%