Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, Ta có: 24nMg + 56nFe = 9,2 (g) (1)
\(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
BT e, có: 2nMg + 2nFe = 2nH2 = 0,5 (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{Mg}=0,15\left(mol\right)\\n_{Fe}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,15.24}{9,2}.100\%\approx39,13\%\\\%m_{Fe}\approx60,87\%\end{matrix}\right.\)
b, BTNT H, có: \(n_{HCl}=2n_{H_2}=0,5\left(mol\right)\Rightarrow C_{M_{HCl}}=\dfrac{0,5}{0,2}=2,5\left(M\right)\)

\(a.BTNT\left(H\right):n_{HCl}=2n_{H_2}=0,65\left(mol\right)\\ \Rightarrow CM_{HCl}=\dfrac{0,65}{0,5}=1,3M\\ b.2Al+6HCl\rightarrow2AlCl_3+3H_2\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ Đặt:\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}\dfrac{3}{2}x+y=0,325\\27x+56y=9,65\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=0,15\\y=0,1\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{Al}=4,05\left(g\right)\\m_{Fe}=5,6\left(g\right)\end{matrix}\right.\)

2.
a)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(n_{H2}=\frac{11,2}{22,4}=0,5\left(mol\right)\)
Gọi a là số mol Fe b là số mol Zn\(\left\{{}\begin{matrix}56a+65b=29,8\\a+b=0,5\end{matrix}\right.\rightarrow\left\{{}\begin{matrix}a=0,3\\b=0,2\end{matrix}\right.\)
\(\%m_{Fe}=\frac{0,3.56}{29,8}.100\%=56,38\%\)
\(\%m_{Zn}=100\%=56,38\%=43,62\%\)
b)
\(n_{HCl}=0,5.2=1\left(mol\right)\)
\(CM_{HCl}=\frac{1}{0,6}=\frac{5}{2}M\)
1.
a,\(2Fe+3Cl_2\underrightarrow{^{to}}2FeCl_3\)
\(n_{Fe}=\frac{1,68}{56}=0,03\left(mol\right)\)
\(n_{Cl2}=\frac{0,84}{22,4}0,0375\left(mol\right)\)
Lập tỉ lệ : \(\frac{n_{Fe}}{2}=0,015< \frac{n_{CL2}}{3}=0,0125\)
Vậy Cl2 hết Fe dư
\(n_{Fe_{du}}=n_{Fe}-\frac{2}{3}n_{Cl2}\)
\(=0,03-\frac{2}{3}.0,0375=0,005\left(mol\right)\)
\(\rightarrow m_{Fe_{du}}=0,005.56=0,28\left(g\right)\)
\(n_{FeCl3}=\frac{2}{3}n_{Cl2}=0,025\left(mol\right)\)
\(\rightarrow m_{FeCl3}=0,025.162,5=4,065\left(g\right)\)
b, \(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,005___0,01__________
\(\rightarrow n_{HCl}=0,01\left(mol\right)\)
\(V_{HCl}=\frac{0,01}{0,5}=0,02\left(l\right)\)

1.
a )\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(n_{H2}=\frac{5,6}{22,4}=0,25\left(mol\right)\)
\(n_{Zn}=n_{H2}=0,25\left(mol\right)\)
\(\rightarrow,m_{Zn}=0,25.65=16,25\left(g\right);m_{Cu}=30-16,25=13,75\left(g\right)\)
b)
\(\%m_{Zn}=\frac{16,25}{30}.100\%=54,17\%\)
\(\%m_{Cu}=100\%-54,17\%=45,83\%\)
c)
\(n_{HCl}=2n_{H2}=0,5\left(mol\right)\)
\(C\%_{HCl}=\frac{0,5.36,5}{200}.100\%=9,125\%\)
2.
a)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(n_{H2}=\frac{3,36}{22,4}=0,15\left(mol\right)\)\(m_{Fe}=0,15.65=8,4\left(g\right),m_{Ag}=15-8,4=6,6\left(g\right)\)
b)
\(\%m_{Fe}=\frac{8,4}{15}.100\%=56\%\)
\(\%m_{Ag}=100\%-56\%=44\%\)
c)
\(n_{HCl}=2n_{H2}=0,3\left(mol\right)\)
\(\rightarrow m_{dd_{HCL}}=\frac{0,3.36,5}{15,6\%}=70,19\left(g\right)\)

\(n_{H_2}=\dfrac{7,84}{22,4}=0,35mol\)
Gọi \(\left\{{}\begin{matrix}n_{Fe}=x\\n_{Zn}=y\end{matrix}\right.\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
x x ( mol )
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}56x+65y=21,4\\x+y=0,35\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,15\\y=0,2\end{matrix}\right.\)
\(\Rightarrow m_{Fe}=0,15.56=8,4g\)
\(\Rightarrow m_{Zn}=0,2.65=13g\)
\(\%m_{Fe}=\dfrac{8,4}{21,4}.100=39,25\%\)
\(\%m_{Zn}=100\%-39,25\%=60,75\%\)
\(m_{FeCl_2}=0,15.127=19,05g\)
\(m_{ZnCl_2}=0,2.136=27,2g\)

Gọi \(\left\{{}\begin{matrix}n_{Ag}=a\left(mol\right)\\n_{FeO}=b\left(mol\right)\end{matrix}\right.\)
\(n_{SO_2}=\dfrac{1,344}{22,4}=0,6\left(mol\right)\)
PTHH:
\(2Ag+2H_2SO_4\rightarrow Ag_2SO_4+SO_2\uparrow+2H_2O\)
a a \(\dfrac{a}{2}\) \(\dfrac{a}{2}\)
\(2FeO+4H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+SO_2\uparrow+4H_2O\)
b 2b \(\dfrac{b}{2}\) \(\dfrac{b}{2}\)
Hệ pt
\(\left\{{}\begin{matrix}108a+72b=11,52\\\dfrac{a}{2}+\dfrac{b}{2}=0,06\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,08\left(mol\right)\\b=0,04\left(mol\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{Ag}=0,08.108=8,64\left(g\right)\\m_{FeO}=0,04.72=2,88\left(g\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}\%m_{Ag}=\dfrac{8,64}{11,52}=75\%\\\%m_{FeO}=100\%-75\%=25\%\end{matrix}\right.\)
b, \(\rightarrow n_{H_2SO_4}=0,08+0,4.2=0,16\left(mol\right)\\ \rightarrow C_{MddH_2SO_4}=\dfrac{0,16}{0,8}=0,2M\)
c, \(n_{NaOH}=1,25.0,5=0,625\left(mol\right)\)
PTHH:
\(6NaOH+Fe_2\left(SO_4\right)_3\rightarrow2Fe\left(OH\right)_3+3Na_2SO_4\)
LTL: \(\dfrac{0,625}{6}>\dfrac{0,04}{2}\) => NaOH dư
Theo pthh:
\(\left\{{}\begin{matrix}n_{NaOH\left(pư\right)}=6n_{Fe_2\left(SO_4\right)_3}=6.0,04=0,24\left(mol\right)\\n_{Na_2SO_4}=3n_{Fe_2\left(SO_4\right)_3}=3.0,04=0,12\left(mol\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}C_{MddNaOH\left(dư\right)}=\dfrac{0,24}{0,5}=0,48M\\C_{MddNa_2SO_4}=\dfrac{0,12}{0,5}=0,24M\end{matrix}\right.\)

1)
Fe + 2HCl --> FeCl2 + H2
Cu + 2H2SO4 --> CuSO4 + SO2 + 2H2O
2)
- Xét TN1:
\(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
0,15<------------------0,15
=> mFe = 0,15.56 = 8,4 (g)
\(\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{8,4}{14,8}.100\%=56,757\%\\\%m_{Cu}=100\%-56,757\%=43,243\%\end{matrix}\right.\)
3)
- Xét TN2:
\(n_{Cu}=\dfrac{29,6.43,243\%}{64}=0,2\left(mol\right)\)
PTHH: Cu + 2H2SO4 --> CuSO4 + SO2 + 2H2O
0,2-------------------------->0,2
=> V = 0,2.22,4 = 4,48 (l)

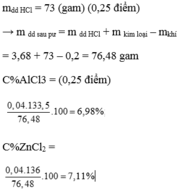
nH2 = 0,5 mol
Đặt nFe = x
nZn = y
Fe + 2HCl → FeCl2 + H2 (1)
x......2x...........x.............x
Zn + 2HCl → ZnCl2 + H2 (2)
y........2y..............y........y
Từ (1)(2) ta có hệ
\(\left\{{}\begin{matrix}56x+65y=29,8\\x+y=0,5\end{matrix}\right.\)
⇒ \(\left\{{}\begin{matrix}x=0,3\\y=0,2\end{matrix}\right.\)
⇒ %Fe = \(\dfrac{0,3.56.100\%}{29,8}\)\(\approx\)56,38%
⇒ %Zn = \(\dfrac{0,2.65.100\%}{29,8}\)\(\approx\) 43,62%
⇒ CM HCl = \(\dfrac{1}{0,6}\) = \(\dfrac{5}{3}\) (M)