Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a,VddC2H5OH=23+27=50(ml)
Đr=\(\dfrac{23}{50}\).100=46o
b,mC2H5OH=23.0,8=18,4(g)
nC2H5OH=\(\dfrac{18,4}{46}\)=0,4(mol)
PTHH: 2C2H5OH + 2K ---> 2C2H5OK + H2
0,4-------------------------------------->0,2
=> VH2 = 0,2.24 = 4,8 (l
a,VddC2H5OH=23+27=50(ml)
Đr=2350.100=46ob, mC2H5OH=23.0,8=18,4(g)
nC2H5OH=18,446=0,4(mol)a,
VddC2H5OH=23+27=50(ml)Đr=2350.100=46ob,
mC2H5OH=23.0,8=18,4(g)
nC2H5OH=18,446=0,4(mol)
PTHH: 2C2H5OH + 2K ---> 2C2H5OK + H2
0,4-------------->0,2
=> VH2 = 0,2.24 = 4,8 (l)

\(a,V_{C_2H_5OH}=\dfrac{10.96}{100}=9,6\left(ml\right)\\ m_{C_2H_5OH}=9,6.0,8=7,68\left(g\right)\\ n_{C_2H_5OH}=\dfrac{7,68}{46}=\dfrac{96}{575}\left(mol\right)\)
PTHH: 2C2H5OH + 2Na ---> 2C2H5ONa + H2
\(\dfrac{96}{575}\)------------------------------------->\(\dfrac{48}{575}\)
\(V_{H_2}=\dfrac{48}{575}.22,4=1,87\left(l\right)\)
\(b,V_{dd}=12+10,6=20,6\left(ml\right)\\ Đ_r=\dfrac{9,6}{20,6}.100=46,6^o\)

nH2 = 85,12 : 22,4 = 3,8 (mol) ; nH2O = VH2O.D = 108 (g) => nH2O = 108/18 = 6 (mol)
PTHH:
2Na + 2C2H5OH → 2C2H5ONa + H2↑
x → 0,5x (mol)
2Na + 2H2O → 2NaOH + H2↑
6 → 3 (mol)
Ta có: nH2 = 0,5x + 3 = 3,8
=> x = 1,6 (mol) = nC2H5OH
mC2H5OH = 1,6.46 = 73,6 (g)
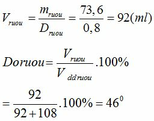

\(a,n_{C_6H_{12}O_6}=\dfrac{36}{180}=0,2\left(mol\right)\)
PTHH: \(C_6H_{12}O_6\underrightarrow{\text{men rượu}}2C_2H_5OH+2CO_2\uparrow\)
0,2----------------->0,4----------->0,4
=> VCO2 = 0,4.22,4 = 8,96 (l)
b, mC2H5OH = 0,4.46.50% = 9,2 (g)
\(c,V_{C_2H_5OH}=\dfrac{9,2}{0,8}=11,5\left(ml\right)\\ \rightarrow V_{ddC_2H_5OH}=\dfrac{11,5.100}{60}=\dfrac{115}{6}\left(ml\right)\)

nC2H5OH=0,5(mol)
V(C2H5OH)=23/0,8=28,75(ml)
=> A=Dr= (28,75/250).100=11,5o
PTHH: C2H5OH + O2 -men giấm---> CH3COOH + H2O
nCH3COOH=C2H5OH=0,5(mol)
=>mCH3COOH=0,5. 60=30(g)
=> m(giấm ăn)= 30/5%=600(g)
=>a=600(g)

\(a,V_{C_2H_5OH}=\dfrac{96.30}{100}=28,8\left(ml\right)\\ \rightarrow m_{C_2H_5OH}=28,8.0,8=23,04\left(ml\right)\\ \rightarrow n_{C_2H_5OH}=\dfrac{23,04}{46}=0,5\left(mol\right)\)
PTHH: 2C2H5OH + 2Na ---> 2C2H5ONa + H2
0,5----------------------------------->0,25
\(\rightarrow V_{H_2}=0,25.22,4=5,6\left(l\right)\)
b, \(n_{CH_3COOH}=\dfrac{36}{60}=0,6\left(mol\right)\)
PTHH: \(C_2H_5OH+CH_3COOH\xrightarrow[t^o]{H_2SO_{4\left(đ\right)}}CH_3COOC_2H_5+H_2O\)
LTL: 0,5 < 0,6 => CH3COOH dư
Theo pthh: nCH3COOH = nC2H5OH = 0,5 (mol)
=> meste = 0,5.88.70% = 30,8 (g)

Gọi số mol C2H5OH, H2O lần lượt là x, y
m dd rượu = mC2H5OH + mH2O
→ 46x + 18y = 8,2 (g)
nH2 = \(\frac{3,36}{22,4}\) = 0,15 mol(1)
2C2H5OH + 2Na → 2C2H5ONa + H2↑
x_____________________________\(\frac{x}{2}\)
2H2O + 2Na → 2NaOH + H2↑
y_____________________\(\frac{y}{2}\)
\(n_{H2}=\frac{x}{2}+\frac{y}{2}=0,15\left(mol\right)\left(2\right)\)
Từ (1) và (2) → x = 0,1; y = 0,2
→ Trong 8,2 g dd rượu có 0,2 . 18 = 3,6 (g) H2O
V dd rượu =\(\frac{8,2}{0,82}\)= 10 (ml)
VH2O trong rượu = \(\frac{3,6}{1}\) = 3,6 (ml)
Trong 10ml rượu etylic có 10-3,6 = 6,4 (ml) C2H5OH
→ Trong 100ml rượu etylic có \(\frac{100.6,4}{10}\) = 64 (ml) C2H5OH
Vậy độ rượu là \(m^o=64^o\)

a. \(V_{dd}=92+108=200\left(ml\right)\)
\(Đ_{rượu}=\dfrac{92}{200}.100=46^o\)
b.\(V_{dd\left(15^o\right)}=\dfrac{92.100}{11,5}=800\left(ml\right)\)
\(V_{H_2O\left(thêm\right)}=800-200=600\left(ml\right)\)
c.\(V_{rượu\left(23^o\right)}=\dfrac{100.23}{100}=23\left(ml\right)\)
\(V_{rượu\left(sau\right)}=23+92=115\left(ml\right)\)
\(V_{dd\left(sau\right)}=800+100=900\left(ml\right)\)
\(Đ_{rượu}=\dfrac{115}{900}.100=12,78^o\)
\(a,V_{ddC_2H_5OH}=23+27=50\left(ml\right)\\ Đ_r=\dfrac{23}{50}.100=46^o\\ b,m_{C_2H_5OH}=23.0,8=18,4\left(g\right)\\ n_{C_2H_5OH}=\dfrac{18,4}{46}=0,4\left(mol\right)\)
PTHH: 2C2H5OH + 2K ---> 2C2H5OK + H2
0,4-------------------------------------->0,2
=> VH2 = 0,2.24 = 4,8 (l)