Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 19 :
\(a) n_{Al} = \dfrac{10,8}{27} = 0,4(mol)\\ 2Al + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2\\ n_{H_2} = \dfrac{3}{2}n_{Al} = 0,6(mol)\\ V_{H_2} = 0,6.22,4 = 13,44(lít)\\ b) \text{Chất tan : }Al_2(SO_4)_3\\ n_{Al_2(SO_4)_3} = \dfrac{1}{2}n_{Al} = 0,2(mol)\\ m_{Al_2(SO_4)_3} = 0,2.342 = 68,4(gam)\)
Bài 18 :
\(a) n_{HCl} = \dfrac{250.7,3\%}{36,5 } = 0,5(mol)\\ Zn + 2HCl \to ZnCl_2 + H_2\\ n_{H_2} = \dfrac{1}{2}n_{HCl} = 0,25(mol) \Rightarrow V_{H_2} = 0,25.22,4 = 5,6(lít)\\ b) \text{Chất tan : } ZnCl_2\\ n_{ZnCl_2} = n_{H_2} = 0,25(mol)\\ m_{ZnCl_2} = 0,25.136 = 34(gam)\)

\(n_{Zn}=\dfrac{6.5}{65}=0.1\left(mol\right)\)
\(n_{H_2SO_4}=0.3\cdot1=0.3\left(mol\right)\)
\(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
\(0.1........0.1...........0.1.......0.1\)
\(\Rightarrow H_2SO_4dư\)
\(n_{H_2SO_4\left(dư\right)}=0.3-0.1=0.2\left(mol\right)\)
\(n_{ZnSO_4}=n_{H_2}=0.1\left(mol\right)\)
\(C_{M_{ZnSO_4}}=\dfrac{0.1}{0.3}=0.33\left(M\right)\)
\(C_{M_{H_2SO_4\left(dư\right)}}=\dfrac{0.2}{0.3}=0.66\left(M\right)\)
\(a) Zn + H_2SO_4 \to ZnSO_4 + H_2\\ b) n_{Zn} = \dfrac{6,5}{65} = 0,1 < n_{H_2SO_4} =0,3 \to H_2SO_4\ dư\\ n_{H_2SO_4\ pư} = n_{ZnSO_4} = n_{Zn} = 0,1(mol)\\ n_{H_2SO_4\ dư} = 0,3 - 0,1 = 0,2(mol)\\ c) C_{M_{ZnSO_4}} = \dfrac{0,1}{0,3} = 0,33M\\ C_{M_{H_2SO_4}} = \dfrac{0,2}{0,3} = 0,67M\)

PTHH: \(BaCl_2+H_2SO_4\rightarrow2HCl+BaSO_4\downarrow\)
a+b) Ta có: \(n_{BaCl_2}=\dfrac{400\cdot5,2\%}{208}=0,1\left(mol\right)=n_{H_2SO_4}=n_{BaSO_4}\)
\(\Rightarrow\left\{{}\begin{matrix}m_{ddH_2SO_4}=\dfrac{0,1\cdot98}{20\%}=49\left(g\right)\\m_{BaSO_4}=0,1\cdot233=23,3\left(g\right)\end{matrix}\right.\)
c) Theo PTHH: \(n_{HCl}=0,2\left(mol\right)\) \(\Rightarrow m_{HCl}=0,2\cdot36,5=7,3\left(g\right)\)
Mặt khác: \(m_{dd\left(sau.p/ứ\right)}=m_{ddBaCl_2}+m_{ddH_2SO_4}-m_{BaSO_4}=425,7\left(g\right)\)
\(\Rightarrow C\%_{HCl}=\dfrac{7,3}{425,7}\cdot100\%\approx1,71\%\)
Bạn xem lại giúp mình , coi đề có bị thiếu gì không nhé

\(n_{KOH}=\dfrac{400.7\%}{56}=0,5\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{100.19,6\%}{98}=0,2\left(mol\right)\)
PTHH: 2KOH + H2SO4 --> K2SO4 + 2H2O
Xét tỉ lệ: \(\dfrac{0,5}{2}>\dfrac{0,2}{1}\) => KOH dư, H2SO4 hết
PTHH: 2KOH + H2SO4 --> K2SO4 + 2H2O
0,4<----0,2-------->0,2
=> \(\left\{{}\begin{matrix}m_{KOH\left(dư\right)}=\left(0,5-0,4\right).56=5,6\left(g\right)\\m_{K_2SO_4}=0,2.174=34,8\left(g\right)\end{matrix}\right.\)
mdd sau pư = 400 + 100 = 500 (g)
=> \(\left\{{}\begin{matrix}C\%_{KOH.dư}=\dfrac{5,6}{500}.100\%=1,12\%\\C\%_{K_2SO_4}=\dfrac{34,8}{500}.100\%=6,96\%\end{matrix}\right.\)

\(n_{KOH}=\dfrac{400.7}{100}:56=0,5\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{100.19,6}{100}:98=0,2\left(mol\right)\)
\(2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O\)
0,4 0,2 0,2
Lập tỉ lệ:
\(\dfrac{0,5}{2}>\dfrac{0,2}{1}\) => KOH dư.
\(m_{dd}=400+100=500\left(g\right)\)
\(n_{KOH.dư}=0,5-0,4=0,1\left(mol\right)\)
\(C\%_{K_2SO_4}=\dfrac{0,2.174.100}{500}=6,96\%\)
\(C\%_{KOH}=\dfrac{0,1.56.100}{500}=1,12\%\)

\(n_{H_2SO_4}\)=24,5:98=0,25(mol)
\(n_{BaCl_2}\)=10,4:208=0,05(mol)
Ta có PTHH:
BaCl2+H2SO4->BaSO4+2HCl
0,05.......0,05......................0,1.....(mol)
Ta có:\(\dfrac{n_{BaCl_2}}{1}\)<\(\dfrac{n_{H_2SO_4}}{1}\)=>BaCl2 hết,H2SO4 dư.Tính theo BaCl2
Sau pư,các chất còn lại là:BaSO4,HCl,H2SO4 dư
Theo PTHH:\(m_{BaSO_4}\)=233.0,05=11,65(g)
mHCl=0,1.36,5=3,65(g)
\(m_{H_2SO_4\left(dư\right)}\)=98.(0,25-0,05)=19,6(g)
PTHH: H2SO4 + BaCl2 ----> BaSO4\(\downarrow\) + 2HCl
n\(H_2SO_4\) = \(\dfrac{24,5}{98}=0,25\left(mol\right)\)
n\(BaCl_2\) = \(\dfrac{10,4}{208}=0,05\left(mol\right)\)
Ta có tỉ lệ: \(\dfrac{0,25}{1}>\dfrac{0,05}{1}\) = > \(H_2SO_4\) dư, \(BaCl_2\) phản ứng hết (tính theo BaCl2 )
Vậy các chất còn lại sau phản ứng gồm có: H2SO4 dư; BaSO4; HCl
Theo PTHH: n\(H_2SO_4\)(p/ứ) = n\(BaCl_2\) = 0,05 (mol)
=> n\(H_2SO_4\)(dư) = n\(H_2SO_4\)(bđ) - n\(H_2SO_4\)(p/ứ) = 0,25 - 0,05 =0,2 (mol)
=> m\(H_2SO_4\)(dư) = 0,2.98 = 19,6 (g)
Theo PTHH: n\(BaSO_4\) = n\(BaCl_2\)=0,05 (mol)
=> m\(BaSO_4\) = 0,05.233 = 11,65 (g)
Theo PTHH: nHCl = 2n\(BaCl_2\) = 2.0,05 = 0,1 (mol)
=> mHCl = 0,1.36,5 = 3,65 (g)

\(a,n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\\ m_{H_2SO_4}=200.19,6\%=39,2\left(g\right)\\ \rightarrow n_{H_2SO_4}=\dfrac{39,2}{98}=0,4\left(mol\right)\)
PTHH: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\uparrow\)
bđ 0,3 0,4
pư 0,3 0,3
spư 0 0,1 0,3 0,3
\(\rightarrow V_{H_2}=0,3.22,4=6,72\left(l\right)\)
\(b,m_{dd}=19,5+200-0,3.2=218,9\left(g\right)\\ \rightarrow\left\{{}\begin{matrix}C\%_{ZnSO_4}=\dfrac{0,3.161}{218,9}.100\%=22,06\%\\C\%_{H_2SO_4\left(dư\right)}=\dfrac{0,1.98}{218,9}.100\%=4,48\%\end{matrix}\right.\)


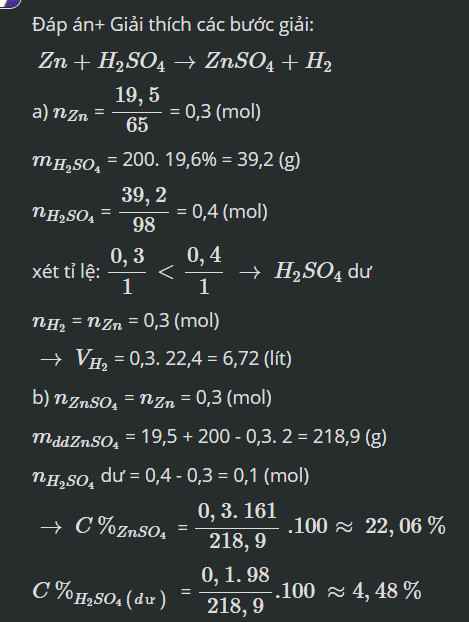
`n_{BaCl_2}={20,8}/{208}=0,1(mol)`
`n_{H_2SO_4}={20.19,6\%}/{98}=0,04(mol)`
`H_2SO_4+BaCl_2->BaSO_4+2HCl`
`0,04->0,04->0,04->0,08(mol)`
Do `0,1>0,04->BaCl_2` dư.
`C\%_{BaCl_2\ du}={208(0,1-0,04)}/{20,8+20-0,04.233}.100\%\approx 39,64\%`
`C\%_{HCl}={0,08.36,5}/{20,8+20-0,04.233}.100\%\approx 9,28\%`