Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

mBaCl2=10.4(g)
nBaCl2=0.05(mol)
mH2SO4=11.76(g)
nH2SO4=0.12(mol)
BaCl2+H2SO4->BaSO4+2HCl
Theo pthh:nH2SO4=nBaCl2
theo bài ra,nH2SO4>nBaCl2
->H2SO4 dư
nH2SO4 dư=0.12-0.05=0.07(mol)
mH2SO4 dư=0.07*98=6.86(g)
nBaSO4=0.05(mol)
mBaSO4=11.65(g)
nHCl=0.05*2=0.1(mol)
mHCl=3.65(g)
mdd sau phản ứng:200+58.8-11.65=247.15(g)
C%(HCl)=3.65:247.15*100=1.48%
C%(H2SO4)=6.86:247.15*100=2.78%

a, \(H_2SO_4+BaCl_2\rightarrow BaSO_{4\downarrow}+2HCl\)
b, Ta có: \(m_{H_2SO_4}=114.20\%=22,8\left(g\right)\Rightarrow n_{H_2SO_4}=\dfrac{22,8}{96}=0,2375\left(mol\right)\)
\(m_{BaCl_2}=400.5,2\%=20,8\left(g\right)\Rightarrow n_{BaCl_2}=\dfrac{20,8}{208}=0,1\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,2375}{1}>\dfrac{0,1}{1}\), ta được H2SO4 dư.
Theo PT: \(n_{BaSO_4}=n_{BaCl_2}=0,1\left(mol\right)\Rightarrow m_{BaSO_4}=0,1.233=23,3\left(g\right)\)
c, Theo PT: \(\left\{{}\begin{matrix}n_{H_2SO_4\left(pư\right)}=n_{BaCl_2}=0,1\left(mol\right)\\n_{HCl}=2n_{BaCl_2}=0,2\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{H_2SO_4\left(dư\right)}=0,2375-0,1=0,1375\left(mol\right)\)
Ta có: m dd sau pư = 114 + 400 - 23,3 = 490,7 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{H_2SO_4\left(dư\right)}=\dfrac{0,1375.98}{490,7}.100\%\approx2,75\%\\C\%_{HCl}=\dfrac{0,2.36,5}{490,7}.100\%\approx1,49\%\end{matrix}\right.\)

Bài 1:
a, Hiện tượng: Có khí mùi hắc thoát ra.
b, Ta có: \(m_{H_2SO_4}=100.24,5\%=24,5\left(g\right)\Rightarrow n_{H_2SO_4}=\dfrac{24,5}{98}=0,25\left(mol\right)\)
PT: \(Na_2SO_3+H_2SO_4\rightarrow Na_2SO_4+SO_2+H_2O\)
Theo PT: \(n_{Na_2SO_3}=n_{H_2SO_4}=0,25\left(mol\right)\)
\(\Rightarrow C\%_{Na_2SO_3}=\dfrac{0,25.126}{200}.100\%=15,75\%\)
c, Theo PT: \(n_{SO_2}=n_{Na_2SO_4}=n_{H_2SO_4}=0,25\left(mol\right)\)
⇒ m dd sau pư = 200 + 100 - 0,25.64 = 284 (g)
\(\Rightarrow C\%_{Na_2SO_4}=\dfrac{0,25.142}{284}.100\%=12,5\%\)
Bài 2:
a, Hiện tượng: Xuất hiện kết tủa trắng.
PT: \(BaCl_2+MgSO_4\rightarrow MgCl_2+BaSO_{4\downarrow}\)
b, Ta có: \(m_{BaCl_2}=200.20,8\%=41,6\left(g\right)\Rightarrow n_{BaCl_2}=\dfrac{41,6}{208}=0,2\left(mol\right)\)
Theo PT: \(n_{MgCl_2}=n_{BaSO_4}=n_{MgSO_4}=n_{BaCl_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{ddMgSO_4}=\dfrac{0,2.120}{12\%}=200\left(g\right)\)
c, Ta có: m dd sau pư = 200 + 200 - 0,2.233 = 353,4 (g)
\(\Rightarrow C\%_{MgCl_2}=\dfrac{0,2.95}{353,4}.100\%\approx5,38\%\)

\(A/n_{Na_2SO_4}=0,1.2=0,2mol\\ n_{BaCl_2}=0,2.3=0,6mol\\ Na_2SO_4+BaCl_2\rightarrow BaSO_4+2NaCl\\ \Rightarrow\dfrac{0,2}{1}< \dfrac{0,6}{1}\Rightarrow BaCl_2.dư\\ Na_2SO_4+BaCl_2\rightarrow BaSO_4+2NaCl\)
0,2mol 0,2mol 0,2mol 0,4mol
\(m_{rắn}=m_{BaSO_4}=0,2.233=46,6g\\ B/C_{M_{NaCl}}=\dfrac{0,4}{0,1+0,2}=\dfrac{4}{3}M\\ C_{M_{BaCl_2}}=\dfrac{0,6-0,2}{0,1+0,2}=\dfrac{4}{3}M\)
\(n_{Na_2SO_4}=0,1.2=0,2\left(mol\right);n_{BaCl_2}=0,2.3=0,6\left(mol\right)\\ PTHH:Na_2SO_4+BaCl_2\rightarrow BaSO_4\downarrow+2NaCl\\ Vì:\dfrac{0,2}{1}< \dfrac{0,6}{1}\rightarrow BaCl_2dư\\ n_{BaSO_4}=n_{BaCl_2\left(p.ứ\right)}=n_{Na_2SO_4}=0,2\left(mol\right)\\ a,m_{rắn}=m_{BaSO_4}=233.0,2=46,6\left(g\right)\)
b, Dung dịch sau phản ứng có: NaCl và BaCl2 dư
\(n_{BaCl_2\left(dư\right)}=0,6-0,2=0,4\left(mol\right)\\ n_{NaCl}=0,2.2=0,4\left(mol\right)\\V_{ddsau}=0,1+0,2=0,3\left(mol\right)\\ C_{MddBaCl_2}=\dfrac{0,4}{0,3}=\dfrac{4}{3}\left(M\right);C_{MddNaCl}=\dfrac{0,4}{0,3}=\dfrac{4}{3}\left(M\right)\)

Gọi nNa2CO3 = x (mol)
Na2CO3 + 2HCl \(\rightarrow\) 2NaCl + H2O + CO2
x \(\rightarrow\) 2x \(\rightarrow\) 2 x (mol)
C%(NaCl) = \(\frac{2.58,5x}{200+120}\) . 100% = 20%
=> x =0,547 (mol)
mNa2CO3 = 0,547 . 106 = 57,982 (g)
mHCl = 2 . 0,547 . 36,5 =39,931 (g)
C%(Na2CO3) =\(\frac{57,892}{200}\) . 100% = 28,946%
C%(HCl) = \(\frac{39,931}{120}\) . 100% = 33,28%
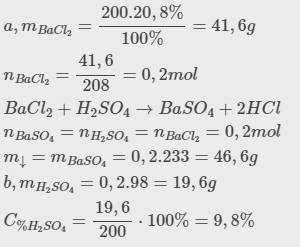
\(m_{BaCl_2}=\dfrac{200.5,2}{100}=10,4\left(g\right)\\ \rightarrow n_{BaCl_2}=\dfrac{10,4}{208}=0,05\left(mol\right)\)
\(m_{H_2SO_4}=\dfrac{58,8.20}{100}=11,76\left(g\right)\\ \rightarrow n_{H_2SO_4}=\dfrac{11,76}{98}=0,12\left(mol\right)\)
\(PTHH:BaCl_2+H_2SO_4\rightarrow BaSO_4\downarrow+2HCl\)
- Ta có: 0,05/1 < 0,12/1
=> BaCl2 hết, H2SO4 dư.
=> Các chất trong dd sau phản ứng là H2SO4 (dư) và HCl.
\(n_{BaSO_4}=n_{BaCl_2}=0,05\left(mol\right)\\ \rightarrow m_{BaSO_4}=0,05.233=11,65\left(g\right)\)
Ta có: \(m_{ddsau}=200+58,8-11,65=247,15\left(g\right)\)
\(m_{H_2SO_4\left(dư\right)}=11,76-\left(0,12-0,05\right).98=4,9\left(g\right)\)
\(n_{HCl}=2.0,05=0,1\left(mol\right)\\ \rightarrow m_{HCl}=0,1.36,5=3,65\left(g\right)\)
=> \(C\%_{ddH_2SO_4\left(dư\right)}=\dfrac{4,9}{247,15}.100\approx1,983\%\)
\(C\%ddHCl=\dfrac{3,65}{217,15}.100\approx1,477\%\)