Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

- Đặt \(\left\{{}\begin{matrix}n_{C_2H_5OH}=a\left(mol\right)\\n_{CH_3COOH}=b\left(mol\right)\end{matrix}\right.\) \(\Rightarrow46a+60b=33,2\left(1\right)\)
\(n_{H_2}=\dfrac{V}{22,4}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
a) \(2C_2H_5OH+2Na\rightarrow2C_2H_5ONa+H_2\)
2 2 1 (mol)
a a a/2 (mol)
\(2CH_3COOH+2Na\rightarrow2CH_3COONa+H_2\)
2 2 1 (mol)
b b b/2 (mol)
Từ hai PTHH trên ta có: \(\dfrac{a}{2}+\dfrac{b}{2}=n_{H_2}=0,3\Rightarrow a+b=0,6\left(2\right)\)
(1), (2) ta có hệ phương trình: \(\left\{{}\begin{matrix}46a+60b=33,2\\a+b=0,6\end{matrix}\right.\)
Giải ra ta được: \(a=0,2\left(mol\right);b=0,4\left(mol\right)\)
b) \(m_{C_2H_5OH}=n.M=0,2\times46=9,2\left(g\right)\)
\(m_{CH_3COOH}=n.M=0,4\times60=24\left(g\right)\)
c) \(m_{C_2H_5ONa}=n.M=0,2\times68=13,6\left(g\right)\)
\(m_{CH_3COONa}=n.M=0,4\times82=32,8\left(g\right)\)

nH2 = 85,12 : 22,4 = 3,8 (mol) ; nH2O = VH2O.D = 108 (g) => nH2O = 108/18 = 6 (mol)
PTHH:
2Na + 2C2H5OH → 2C2H5ONa + H2↑
x → 0,5x (mol)
2Na + 2H2O → 2NaOH + H2↑
6 → 3 (mol)
Ta có: nH2 = 0,5x + 3 = 3,8
=> x = 1,6 (mol) = nC2H5OH
mC2H5OH = 1,6.46 = 73,6 (g)
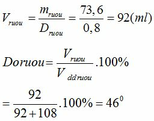

Đáp án: A
Vì dung dịch rượu gồm rượu etylic và nước nên ta gọi:
n H 2 O = x m o l và n C 2 H 5 O H = y m o l
PTHH:
2 N a + 2 H 2 O → 2 N a O H + H 2 ↑ ( 1 )
x mol → 0,5.x mol
2 N a + 2 C 2 H 5 O H → 2 C 2 H 5 O N a + H 2 ↑
y mol → 0,5.y mol
Ta có hệ phương trình:
18 x + 46 y = 10 , 1 0 , 5 x + 0 , 5 y = 0 , 125 ⇒ x = 0 , 05 y = 0 , 2
V C 2 H 5 O H nguyên chất = m D = 0 , 2 . 46 0 , 8 = 11 , 5 m l
V H 2 O = m D = 10 , 1 - 9 , 2 1 = 0 , 9 m l
=> V d d r ư ợ u = V H 2 O + V C 2 H 5 O H = 0,9 + 11,5 = 12,4 ml
=> Độ rượu D 0 = V C 2 H 5 O H V d d r u o u . 100 = 11 , 5 12 , 4 . 100 = 92 , 74 0

\(m_{C_2H_5OH\left(nguyên.chất\right)}=\dfrac{0,5.30}{100}=0,15l=150ml\)
\(\rightarrow m_{H_2O}=500-150=350ml\)
\(m_{C_2H_5OH}=150.0,8=120g\)
\(m_{H_2O}=350.1=350g\)
\(\left\{{}\begin{matrix}n_{C_2H_5OH}=\dfrac{120}{46}=2,6mol\\n_{H_2O}=\dfrac{350}{18}=19,44mol\end{matrix}\right.\)
\(2C_2H_5OH+2Na\rightarrow2C_2H_5ONa+H_2\)
2,6 1,3 ( mol )
\(2Na+2H_2O\rightarrow2NaOH+H_2\)
19,44 9,72 ( mol )
\(V_{H_2}=\left(1,3+9,72\right).22,4=246,848l\)
\(0,5lít=500ml\)
\(m_{C_2H_5OH}=500.0,8=400g\)
\(n_{C_2H_5OH}=\dfrac{400}{46}=8,69mol\)
\(n_{Na}=\dfrac{300}{23}=12,04mol\)
\(2C_2H_5OH+2Na\rightarrow2C_2H_5ONa+H_2\)
8,69 < 12,04 ( mol )
8,69 8,69 ( mol )
\(V_{H_2}=8,96.22,4=200,704l\)

n C2H5OH =a (mol) ; n CH3COOH = b(mol)
=> 46a + 60b = 27,2(1)
$2C_2H_5ONa + 2Na \to 2C_2H_5ONa + H_2$
$2CH_3COOH + 2Na \to 2CH_3COONa + H_2$
Theo PTHH :
n H2 = 0,5a + 0,5b = 5,6/22,4 = 0,25(2)
Từ (1)(2) suy ra a = 0,2 ; b = 0,3
Suy ra:
m C2H5OH = 0,2.46 = 9,2(gam)
m CH3COOH = 0,3.60 = 18(gam)

Gọi \(\left\{{}\begin{matrix}n_{C_2H_5OH}=a\left(mol\right)\\n_{H_2O}=b\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
PTHH:
2C2H5OH + 2Na ---> 2C2H5ONa + H2
a --------------------------------------------> 0,5a
2H2O + 2Na ---> 2NaOH + H2
b --------------------------------> 0,5b
Hệ pt \(\left\{{}\begin{matrix}46a+18b=20,2\\0,5a+0,5b=0,25\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,4\left(mol\right)\\b=0,1\left(mol\right)\end{matrix}\right.\)
\(\rightarrow\left\{{}\begin{matrix}m_{C_2H_5OH}=0,4.46=18,4\left(g\right)\\m_{H_2O}=0,1.18=1,8\left(g\right)\end{matrix}\right.\rightarrow\left\{{}\begin{matrix}V_{C_2H_5OH}=\dfrac{18,4}{0,8}=23\left(ml\right)\\V_{H_2O}=\dfrac{1,8}{1}=1,8\left(ml\right)\end{matrix}\right.\)
=> Độ rượu là: \(\dfrac{23}{23+1,8}=92,74^o\)

\(n_{Mg}=\dfrac{7,2}{24}=0,3\left(mol\right)\\ n_{Cl_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\\ Mg+Cl_2\rightarrow\left(t^o\right)MgCl_2\\ a,Vì:\dfrac{0,2}{1}< \dfrac{0,3}{1}\Rightarrow Mgdư\\n_{Mg\left(p.ứ\right)}=n_{MgCl_2}=n_{Cl_2}=0,2\left(mol\right)\\ \Rightarrow n_{Mg\left(dư\right)}=0,3-0,2=0,1\left(mol\right)\\ m_{Mg\left(dư\right)}=0,1.24=2,4\left(g\right)\\ b,m_{MgCl_2}=0,2.95=19\left(g\right)\)
\(n_{Mg}=\dfrac{m}{M}=\dfrac{7,2}{24}=0,3\) (mol)
\(n_{Cl_2}=\dfrac{V}{22,4}=\dfrac{4,48}{22,4}=0,2\)(mol)
PTHH : Mg + Cl2 ---> MgCl2
1 : 1 : 1
Dễ thấy : \(\dfrac{n_{Mg}}{1}>\dfrac{n_{Cl_2}}{1}\)
=> Mg dư 0,1 mol
=> \(m_{Mg}=n.M=0,1.24=2,4\left(g\right)\)
=> \(n_{MgCl_2}=0,2\left(mol\right)\) => \(m_{MgCl_2}=n.M=0,2.\left(24+71\right)=19\left(g\right)\)
\(n_{Mg}=\dfrac{m}{M}=\dfrac{7,2}{24}=0,3\) (mol)
\(n_{Cl_2}=\dfrac{V}{22,4}=\dfrac{4,48}{22,4}=0,2\)(mol)
PTHH : Mg + Cl2 ---> MgCl2
1 : 1 : 1
Dễ thấy : \(\dfrac{n_{Mg}}{1}>\dfrac{n_{Cl_2}}{1}\)
=> Mg dư 0,1 mol
=> \(m_{Mg}=n.M=0,1.24=2,4\left(g\right)\)
=> \(n_{MgCl_2}=0,2\left(mol\right)\) => \(m_{MgCl_2}=n.M=0,2.\left(24+71\right)=19\left(g\right)\)

Ta có: \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PT: \(2Na+2C_2H_5OH\rightarrow2C_2H_5ONa+H_2\)
Theo PT: \(n_{C_2H_5OH}=2n_{H_2}=0,3\left(mol\right)\)
\(\Rightarrow m_{C_2H_5OH}=0,3.46=13,8\left(g\right)=m\)

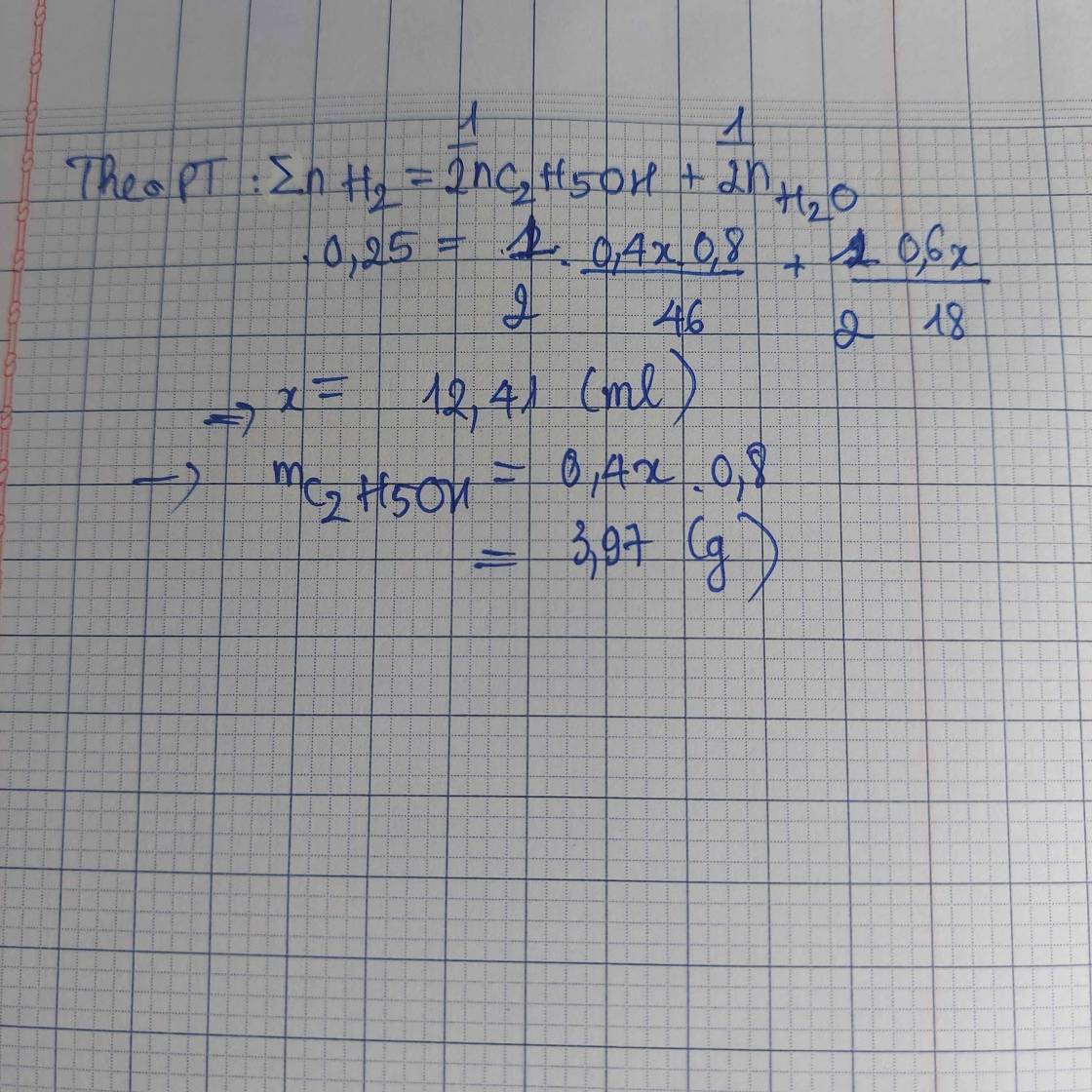
nC2H5OH=0,3(mol)
PTHH: C2H5OH + Na -> C2H5ONa + 1/2 H2
nH2=nC2H5OH/2=0,3/2=0,15(mol)
=>V(H2,đktc)=0,15.22,4=3,36(l)