Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Gọi \(\left\{{}\begin{matrix}n_{Zn}=a\left(mol\right)\\n_{Fe}=b\left(mol\right)\\n_{Al}=c\left(mol\right)\end{matrix}\right.\) => 65a + 56b + 27c = 10,65 (1)
PTHH: Zn + 2HCl --> ZnCl2 + H2
Fe + 2HCl --> FeCl2 + H2
2Al + 6HCl --> 2AlCl3 + 3H2
=> \(n_{H_2}=a+b+1,5c=\dfrac{5,04}{22,4}=0,225\left(mol\right)\) (2)
PTHH: Zn + Cl2 --to--> ZnCl2
2Fe + 3Cl2 --to--> 2FeCl3
2Al + 3Cl2 --to--> 2AlCl3
=> \(n_{Cl_2}=a+1,5b+1,5c=\dfrac{5,6}{22,4}=0,25\left(mol\right)\) (3)
(1)(2)(3) => \(\left\{{}\begin{matrix}a=0,1\left(mol\right)\\b=0,05\left(mol\right)\\c=0,05\left(mol\right)\end{matrix}\right.\) => \(\left\{{}\begin{matrix}m_{Zn}=0,1.65=6,5\left(g\right)\\m_{Fe}=0,05.56=2,8\left(g\right)\\m_{Al}=0,05.27=1,35\left(g\right)\end{matrix}\right.\)
a) \(\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{6,5}{10,65}.100\%=61,033\%\\\%m_{Fe}=\dfrac{2,8}{10,65}.100\%=26,291\%\\\%m_{Al}=\dfrac{1,35}{10,65}.100\%=12,676\%\end{matrix}\right.\)
b) nHCl = 2a + 2b + 3c = 0,45 (mol)
=> mHCl = 0,45.36,5 = 16,425 (g)
=> \(a\%=C\%=\dfrac{16,425}{200}.100\%=8,2125\%\)
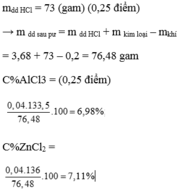
c) mdd sau pư = 10,65 + 200 - 0,225.2 = 210,2 (g)
=> \(\left\{{}\begin{matrix}C\%_{ZnCl_2}=\dfrac{0,1.136}{210,2}.100\%=6,47\%\\C\%_{FeCl_2}=\dfrac{0,05.127}{210,2}.100\%=3,02\%\\C\%_{AlCl_3}=\dfrac{0,05.133,5}{210,2}.100\%=3,176\%\end{matrix}\right.\)

a) \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PTHH: Zn + H2SO4 --> ZnSO4 + H2
0,2<----------------0,2<---0,2
\(\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{0,2.65}{16,2}.100\%=80,247\%\\\%m_{Cu}=100\%-80,247\%=19,753\%\end{matrix}\right.\)
b) \(m_{ZnSO_4}=0,2.161=32,2\left(g\right)\)

\(a,n_{Al}=x(mol);n_{Fe}=y(mol)\\ \Rightarrow 27x+56y=11(1)\\ n_{H_2}=\dfrac{8,96}{22,4}=0,4(mol)\\ 2Al+6HCl\to 2AlCl_3+3H_2\\ Fe+2HCl\to FeCl_2+H_2\\ \Rightarrow 1,5x+y=0,4(2)\\ (1)(2)\Rightarrow x=0,2(mol);y=0,1(mol)\\ \Rightarrow \begin{cases} \%_{Al}=\dfrac{0,2.27}{11}.100\%=49,09\%\\ \%_{Fe}=100\%-49,09\%=50,91\% \end{cases}\\ b,\Sigma n_{HCl}=3x+2y=0,8(mol)\\ \Rightarrow V_{dd_{HCl}}=\dfrac{0,8}{2}=0,4(l)\)

\(n_{H_2}=\dfrac{7,84}{22,4}=0,35mol\)
Gọi \(\left\{{}\begin{matrix}n_{Fe}=x\\n_{Zn}=y\end{matrix}\right.\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
x x ( mol )
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}56x+65y=21,4\\x+y=0,35\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,15\\y=0,2\end{matrix}\right.\)
\(\Rightarrow m_{Fe}=0,15.56=8,4g\)
\(\Rightarrow m_{Zn}=0,2.65=13g\)
\(\%m_{Fe}=\dfrac{8,4}{21,4}.100=39,25\%\)
\(\%m_{Zn}=100\%-39,25\%=60,75\%\)
\(m_{FeCl_2}=0,15.127=19,05g\)
\(m_{ZnCl_2}=0,2.136=27,2g\)

a.\(n_{H_2}=\dfrac{V_{H_2}}{22,4}=\dfrac{8,96}{22,4}=0,4mol\)
Gọi \(\left\{{}\begin{matrix}n_{Fe}=x\\n_{Zn}=y\end{matrix}\right.\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
x x ( mol )
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}56x+65y=25,55\\x+y=0,4\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,05\\y=0,35\end{matrix}\right.\)
\(\Rightarrow m_{Fe}=0,05.56=2,8g\)
\(\Rightarrow m_{Zn}=0,35.65=22,75g\)
\(\%m_{Fe}=\dfrac{2,8}{25,55}.100=10,95\%\)
\(\%m_{Zn}=100\%-10,95\%=89,05\%\)
b.\(n_{HCl}=2.0,05+2.0,35=0,8mol\)
\(C_M=\dfrac{n}{V}\Rightarrow V=\dfrac{n}{C_M}=\dfrac{0,8}{2}=0,4l\)



a, Giả sử: \(\left\{{}\begin{matrix}n_{Fe}=x\left(mol\right)\\n_{Zn}=y\left(mol\right)\end{matrix}\right.\)
⇒ 56x + 65y = 12,1 (1)
Ta có: \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Các quá trình:
\(Fe^0\rightarrow Fe^{+2}+2e\)
x___________ 2x (mol)
\(Zn^0\rightarrow Zn^{+2}+2e\)
y____________ 2y (mol)
\(2H^++2e\rightarrow H_2^0\)
______0,4___0,2 (mol)
Theo ĐLBT mol e, có: 2x + 2y = 0,4 (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,1\left(mol\right)\\y=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,1.56}{12,1}.100\%\approx46,3\%\\\%m_{Zn}\approx53,7\%\end{matrix}\right.\)
b, BTNT Fe và Zn, có: \(\left\{{}\begin{matrix}n_{FeCl_2}=n_{Fe}=0,1\left(mol\right)\\n_{ZnCl_2}=n_{Zn}=0,1\left(mol\right)\end{matrix}\right.\)
⇒ m muối = mFeCl2 + mZnCl2 = 0,1.127 + 0,1.136 = 26,3 (g)
c, BTNT H, có: \(n_{HCl}=2n_{H_2}=0,4\left(mol\right)\)
\(\Rightarrow x=C_{M_{HCl}}=\dfrac{0,4}{0,5}=0,8\left(M\right)\)
Bạn tham khảo nhé!