Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Lập PTHH theo các sơ đồ sau
CaCO3+2HCl-->CaCl2+H2O+CO2
BaCO3+2HCl-->BaCl2+CO2+H2O
K2CO3+2HBr-->2KBr+CO2+H2O
Na2CO3+2HCl-->2NaCl+CO2+H2O
MgCO3+2HNO3-->Mg(NO3)2+CO2+H2O
NaHCO3+HCl-->NaCl+CO2+H2O
NaHCO3+HNO3-->NaNO3+CO2+H2O
2NaHCO3+H2SO4-->Na2SO4+2CO2+2H2O
2Na2CO3+HNO3-->2NaNO3+CO2+H2O
CuCO3+2HCl-->CuCl2+CO2+H2O
CaCO3+2HCl-->CaCl2+H2O+CO2
BaCO3+2HCl-->BaCl2+CO2+H2O
K2CO3+2HBr-->2KBr+CO2+H2O
Na2CO3+2HCl-->2NaCl+CO2+H2O
MgCO3+2HNO3-->Mg(NO3)2+CO2+H2O
NaHCO3+HCl-->NaCl+CO2+H2O
NaHCO3+HNO3-->NaNO3+CO2+H2O
2NaHCO3+H2SO4-->Na2SO4+2CO2+2H2O
Na2CO3+2HNO3-->2NaNO3+CO2+H2O
CuCO3+2HCl-->CuCl2+CO2+H2O

\(n_{BaCO_3}=\dfrac{19.7}{197}=0.1\left(mol\right)\)
\(n_{Ba\left(OH\right)_2}=0.15\cdot1=0.15\left(mol\right)\)
\(n_{MgCO_3}=a\left(mol\right),n_{CaCO_3}=b\left(mol\right)\)
\(\Rightarrow m_A=84a+100b=18.4\left(g\right)\left(1\right)\)
\(MgCO_3+2HCl\rightarrow MgCl_2+CO_2+H_2O\)
\(CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O\)
\(n_{CO_2}=a+b\left(mol\right)\)
TH1 : Không tạo muối axit , Ba(OH)2 dư
\(\Rightarrow n_{CO_2}=n_{BaCO_3}=0.1\left(mol\right)\)
\(\Rightarrow a+b=0.1\left(2\right)\)
\(\left(1\right),\left(2\right):a=-0.525,b=0.625\left(L\right)\)
TH2 : Phản ứng tạo hai muối vừa đủ
\(n_{CO_2}=0.1+\left(0.15-0.1\right)\cdot2=0.2\left(mol\right)\)
\(\Rightarrow a+b=0.1\left(3\right)\)
\(\left(1\right),\left(3\right):a=b=0.1\)
\(\%MgCO_3=\dfrac{8.4}{18.4}\cdot100\%=45.65\%\)
\(\%CaCO_3=54.35\%\)

\(n_{CO_2}=\dfrac{6.72}{22.4}=0.3\left(mol\right)\)
\(n_{KOH}=\dfrac{400\cdot5.6\%}{56}=0.4\left(mol\right)\)
\(n_{K_2CO_3}=a\left(mol\right),n_{KHCO_3}=b\left(mol\right)\)
\(2KOH+CO_2\rightarrow K_2CO_3+H_2O\)
\(KOH+CO_2\rightarrow KHCO_3\)
\(\left\{{}\begin{matrix}2a+b=0.4\\a+b=0.3\end{matrix}\right.\)
\(\Leftrightarrow\left\{{}\begin{matrix}a=0.1\\b=0.2\end{matrix}\right.\)
\(K_2CO_3+BaCl_2\rightarrow BaCO_3+2KCl\)
\(0.1...............................0.1\)
\(m_{BaCO_3}=0.1\cdot197=19.7\left(g\right)\)
Ta có : \(n_{CO2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(n_{KOH}=\dfrac{400.5,6}{56.100}=0,4\left(mol\right)\)
\(\dfrac{n_{KOH}}{n_{CO2}}=\dfrac{0,4}{0,3}=\dfrac{4}{3}\Rightarrow\)Tạo 2 muối
\(CO2+2KOH\rightarrow K2CO3+H2O\)
x---------->2x-------->x(mol)
\(CO2+KOH\rightarrow KHCO3\)
y-------->y------------->(mol)
Theo bài ta có HPT\(\left\{{}\begin{matrix}x+y=0,3\\2x+y=0,4\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
\(K2CO3+BaCl2\rightarrow BaCO3+2KCl\)
0,1--------------------------->0,1(mol)
\(\Rightarrow m=m_{BaCO3}=0,1.197=19,7\left(g\right)\)
Chúc bạn học tốt ^.^

CaCO3 →to CaO + CO2 (1)
MgCO3 →to MgO + CO2 (2)
\(n_{CO_2}=\dfrac{15,4}{44}=0,35\left(mol\right)\)
Gọi \(x,y\) lần lượt là số mol của CaCO3 và MgCO3
Theo PT1: \(n_{CO_2}=n_{CaCO_3}=x\left(mol\right)\)
Theo PT2: \(n_{CO_2}=n_{MgCO_3}=y\left(mol\right)\)
Ta có: \(\left\{{}\begin{matrix}100x+84y=31,8\\x+y=0,35\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,15\\y=0,2\end{matrix}\right.\)
Vậy \(n_{CaCO_3}=0,15\left(mol\right);n_{MgCO_3}=0,2\left(mol\right)\)
Theo PT1: \(n_{CaO}=n_{CaCO_3}=0,15\left(mol\right)\)
\(\Rightarrow m_{CaO}=0,15\times56=8,4\left(g\right)\)
Theo PT2: \(n_{MgO}=n_{MgCO_3}=0,2\left(mol\right)\)
\(\Rightarrow m_{MgO}=0,2\times40=8\left(g\right)\)
\(\Sigma m_{oxit}=m_{CaO}+m_{MgO}=8,4+8=16,4\left(g\right)\)

\(a.MgCO_3-t^{^0}->MgO+CO_2\\ CaCO_3-t^{^0}->CaO+CO_2\\ b.n_{MgCO_3}=a,n_{CaCO_3}=b\\ 84a+100b=26,8\\ 40a+56b=13,6\\ a=0,2;b=0,1\\ \%m_{MgCO_3}=\dfrac{84a}{26,8}.100\%=62,69\%\\ \%m_{CaCO_3}=37,31\%\\ c.V_{CO_2}=22,4\left(a+b\right)=6,72L\)
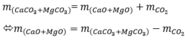

\(Đăt:n_{CO_2}=n_{H_2O}=a\left(mol\right)\)
\(\Rightarrow n_{HCl}=2a\left(mol\right)\)
\(MCO_3+2HCl\rightarrow MCl_2+CO_2+H_2O\)
\(BTKL:\)
\(10+2a\cdot36.5=11.1+44a+18a\)
\(\Rightarrow a=0.1\)
\(V_{CO_2}=0.1\cdot22.4=2.24\left(l\right)\)
cảm ơn ạ