Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 1 :
Giả sử thể tích dung dịch H2SO4 là V ml
\(\rightarrow m_{dd}=1,84V\left(g\right)\rightarrow m_{H2SO4}=1,84V.98\%=1,8032\left(V\right)\)
\(\rightarrow n_{H2SO4}=\frac{1,8032V}{98}=0,0184V\left(mol\right)\)
\(\rightarrow CM_{H2SO4}=\frac{0,0184V.1000}{V}=18,4M\)
\(n_{H2SO4}=2.2,5=5\left(mol\right)\rightarrow m_{H2SO4}=5.98=490\left(g\right)\)
\(\rightarrow m_{dd_{H2SO4_{Can}}}=\frac{490}{98\%}=500\left(g\right)\)
Vậy V dung dịch H2SO4 cần \(=\frac{500}{1,84}=271,74\left(ml\right)\)
Cho 271,74 ml H2SO4 98% vào dung dịch, sau đó thêm H2O vào đủ 2 lít/
Bài 2:
Gọi số mol Na2O cần là x \(\rightarrow m_{Na2O}=62x\)
\(\rightarrow\) m dung dịch sau khi thêm=62x+84,5 gam
\(Na_2O+H_2O\rightarrow2NaOH\)
\(\rightarrow n_{NaOH_{tao.ra}}=2x\rightarrow m_{NaOH_{tao.ra}}=2x.40=80x\left(g\right)\)
\(\rightarrow\) m NaOH trong dung dịch \(=80x+84,5.10\%=80x+8,45\left(g\right)\)
\(\rightarrow C\%_{NaOH}=\frac{\left(80x+8,45\right)}{\left(62x+84,5\right)}=28,45\%\rightarrow x=0,25\)
\(\rightarrow m_{Na2O}=15,5\left(g\right)\)
Bài 3 :
\(n_{MgCO3}=\frac{16,8}{84}=0,2\left(mol\right)\)
\(n_{HCl}=\frac{200.10,95\%}{36,5}=0,6\left(mol\right)\)
\(MgCO_3+2HCl\rightarrow MgCl_2+CO_2+H_2O\)
Nên HCl dư
\(n_{CO2}=0,2\left(mol\right)\)
\(n_{HCl_{du}}=0,6-0,2.2=0,2\left(mol\right)\)
\(m_{dd_{Spu}}=16,8+200-0,2.44=208\left(g\right)\)
\(C\%_{HCl}=\frac{0,2.36,5}{208}.100\%=3,51\%\)
\(C\%_{MgCl2}=\frac{0,2.95}{208}.100\%=9,13\%\)

nNa = 6.9 : 23 = 0.3 mol
4Na + O2 ->2 Na2O
mol : 0.3 -> 0.15
Na2O + H2O -> 2NaOH
mol : 0.15 -> 0.3
mdd = 0.15 x 62 + 140.7 = 150g
C% NaOH = 0.3x40: 150 x 100% = 8%

nBaCl2 = 0.05 mol
nNaOH = 2 mol
BaCl2 + H2SO4 --> BaSO4 + 2HCl
0.05_____0.05______0.05_______0.1
mBaSO4 = 0.05*233 = 11.65 g
NaOH + HCl --> NaCl + H2O
0.1_______0.1
2NaOH + H2SO4 --> Na2SO4 + H2O
2-0.1______0.95
mH2SO4 = 98 g
C%H2SO4 = 98/200*100% = 49%

a/
\(n_{Na_2O}=\dfrac{9,3}{62}=0,15\left(mol\right)\)
\(Na_2O+H_2O\rightarrow2NaOH\)
0,15 0,3 (mol)
\(m_{NaOH}=0,3.40=12\left(g\right)\)
\(m_A=90,7+9,3=100\left(g\right)\)
\(C\%_{NaOH}=\dfrac{12}{100}.100\%=12\%\)
b/
m\(_{FeSO_4}=\dfrac{16.200}{100}=32\left(g\right)\)
\(\rightarrow m_{FeSO_4}=\dfrac{32}{152}=\dfrac{4}{19}\left(mol\right)\)
\(2NaOH+FeSO_4\rightarrow Na_2SO_4+Fe\left(OH\right)_2\downarrow\)
bđ: 0,3 \(\dfrac{4}{19}\) 0 0 (mol)
pư: 0,3 0,15 0,15 0,15 (mol)
dư: 0 \(\dfrac{23}{380}\) (mol)
\(m_{Fe\left(OH\right)_2}=0,15.90=13,5\left(g\right)\)
\(m_C=100+200-13,5=286,5\left(g\right)\)
\(m_{Na_2SO_4}=0,15.142=21,3\left(g\right)\)
\(\rightarrow C\%_{Na_2SO_4}=\dfrac{21,3}{286,5}.100\%\approx7,4\%\)
\(m_{FeSO_4\left(dư\right)}=\dfrac{23}{380}.152=9,2\left(g\right)\)
\(\rightarrow C\%_{FeSO_4\left(dư\right)}=\dfrac{9,2}{286,5}.100\%\approx3,2\%\)

Ta có: \(n_{Al}=\dfrac{3,4}{27}=\dfrac{17}{135}\left(mol\right)\)
PT: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
a, \(n_{H_2}=\dfrac{3}{2}n_{Al}=\dfrac{17}{90}\left(mol\right)\Rightarrow V_{H_2}=\dfrac{17}{90}.22,4=\dfrac{952}{225}\left(l\right)\)
b, \(n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Al}=\dfrac{17}{270}\left(mol\right)\Rightarrow m_{Al_2\left(SO_4\right)_3}=\dfrac{17}{270}.342=\dfrac{323}{15}\left(g\right)\)
c, \(n_{H_2SO_4}=\dfrac{3}{2}n_{Al}=\dfrac{17}{90}\left(mol\right)\Rightarrow C_{M_{H_2SO_4}}=\dfrac{\dfrac{17}{90}}{0,5}=\dfrac{17}{45}\left(M\right)\)

a, \(C\%_{KCl}=\dfrac{20}{20+60}.100\%=25\%\)
b, \(C\%=\dfrac{40}{40+150}.100\%\approx21,05\%\)
c, \(C\%_{NaOH}=\dfrac{60}{60+240}.100\%=20\%\)
d, \(C\%_{NaNO_3}=\dfrac{30}{30+90}.100\%=25\%\)
e, \(m_{NaCl}=150.60\%=90\left(g\right)\)
f, \(m_{ddA}=\dfrac{25}{10\%}=250\left(g\right)\)
g, \(n_{NaOH}=120.20\%=24\left(g\right)\)
Gọi: nNaOH (thêm vào) = a (g)
\(\Rightarrow\dfrac{a+24}{a+120}.100\%=25\%\Rightarrow a=8\left(g\right)\)
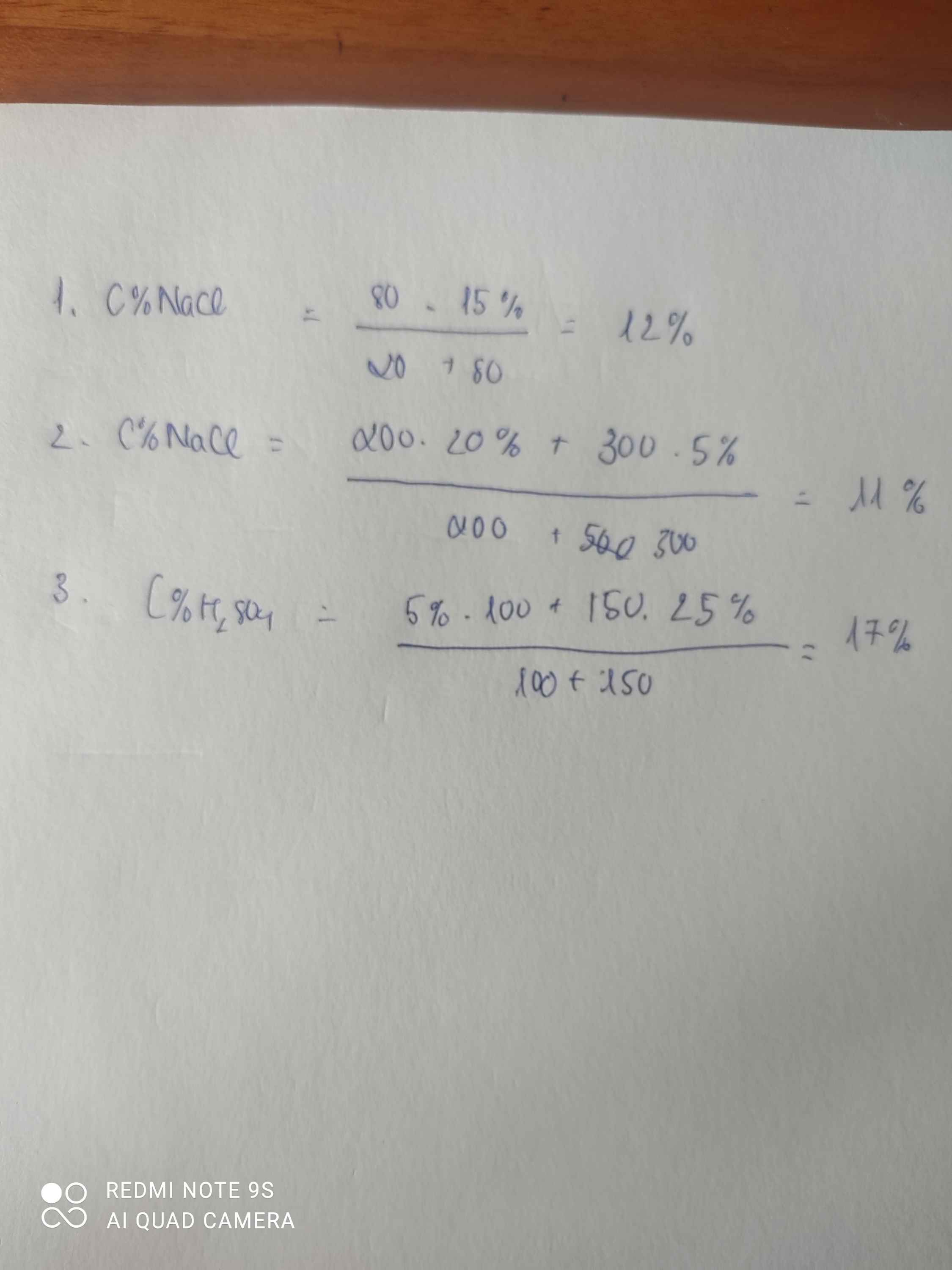


Khối lượng dd NaOH : 1,28*250= 80g => nNaOH = (80*25/100)/40 = 2mol
PT : BaCl2 + H2SO4 ----> BaSO4 + 2HCl
0,05mol --> 0,05mol
H2SO4 + 2NaOH -------> Na2SO4 + 2H2O
1mol <--- 2mol
hoep t trên ta có tổng số mol của H2SO4 : 0,05+1 = 1,05mol => mH2SO4 = 98*1,05 =102,9g
Vậy c%H2SO4 : 102,9/200*100= 51,45%
khối lượng BaCl2 * với 10 ở đâu ra vậy bạn