Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,C_2H_5OH+O_2\left(men.giấm\right)\rightarrow CH_3COOH+H_2O\\ V_{C_2H_5OH\left(ng.chất\right)}=\dfrac{2,875}{10}=0,2875\left(l\right)=287,5\left(ml\right)\\ m_{C_2H_5OH}=287,5.0,8=230\left(g\right)\\ n_{C_2H_5OH}=\dfrac{230}{46}=5\left(mol\right)\\ n_{CH_3COOH\left(LT\right)}=n_{C_2H_5OH}=5\left(mol\right)\\ n_{CH_3COOH\left(TT\right)}=5.80\%=4\left(mol\right)\\ m_{CH_3COOH\left(TT\right)}=4.60=240\left(g\right)\\ b,m_{dd.giấm}=\dfrac{240.100}{5}=4800\left(gam\right)\)

a, \(n_{CH_3COOH}=0,2.1=0,2\left(mol\right)\)
PT: \(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
Theo PT: \(n_{Mg}=n_{H_2}=\dfrac{1}{2}n_{CH_3COOH}=0,1\left(mol\right)\)
\(\Rightarrow m=m_{Mg}=0,1.24=2,4\left(g\right)\)
\(V=V_{H_2}=0,1.22,4=2,24\left(l\right)\)
b, \(C_2H_5OH+O_2\underrightarrow{^{mengiam}}CH_3COOH+H_2O\)
Theo PT: \(n_{C_2H_5OH}=n_{CH_3COOH}=0,2\left(mol\right)\)
\(\Rightarrow m_{C_2H_5OH}=0,2.46=9,2\left(g\right)\)
\(\Rightarrow V_{ddC_2H_5OH}=\dfrac{9,2}{0,8}=11,5\left(ml\right)\)

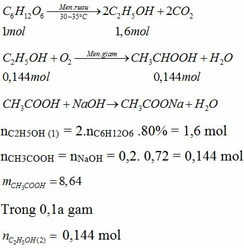
a.\(V_{C_2H_5OH}=\dfrac{10.8}{100}=0,8ml\)
\(m_{C_2H_5OH}=0,8.0,8=1,6g\)
\(n_{C_2H_5OH}=\dfrac{1,6}{46}=0,034mol\)
\(C_2H_5OH+O_2\rightarrow\left(men.giấm\right)CH_3COOH+H_2O\)
0,034 0,034 ( mol )
\(m_{CH_3COOH}=0,034.60.80\%=1,632g\)
b.\(m_{dd_{CH_3COOH}}=\dfrac{1,632}{5\%}=32,64g\)

a)
$C_2H_5OH + O_2 \xrightarrow{men\ giấm} CH_3COOH + H_2O$
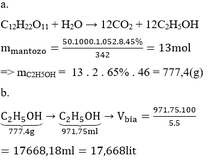
V rượu = 57,5.12/100 = 6,9(lít) = 6900(cm3)
=> m rượu = 6900.0,8 = 5520(gam)
Theo PTHH :
n CH3COOH = n C2H5OH = 5520/46 = 120(mol)
m CH3COOH = 120.60 = 7200(gam)
b)
m dd giấm = 7200/4% = 180 000(gam)
\(V_r=57.5\cdot0.12=6.9\left(l\right)\)
\(m_{C_2H_5OH}=6.9\cdot0.8=5.52\left(g\right)\)
\(n_{C_2H_5OH}=\dfrac{5.52}{46}=0.12\left(mol\right)\)
\(n_{C_2H_5OH\left(pư\right)}=0.12\cdot92\%=0.1104\left(mol\right)\)
\(C_2H_5OH+O_2\underrightarrow{mg}CH_3COOH+H_2O\)
\(0.1104........................0.1104\)
\(m_{dd_{CH_3COOH}}=\dfrac{0.1104\cdot60}{4\%}=165.6\left(g\right)\)

Câu 16 :
1)
1 lít = 1000ml
V rượu = 1000.92/100 = 920(ml)
=> m C2H5OH = D.V = 0,8.920 = 736(gam)
2)
$C_2H_5OH \xrightarrow{t^o,xt} C_2H_4 + H_2O$
n C2H4 = n C2H5OH = 736/46 = 16(mol)
V C2H4 = 16.22,4 = 358,4 lít