Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

vậy phải kí hiệu là D mới đúng á bạn!
Bài làm
m(NaOh)= 8g => n(Naoh)= m/M= 8/40=0,2 mol
m(H2O)=32g; D(H2O)= 1g/ml; D( dd Naoh)=1,25g/ml
Giải
m(dd)= 8+32=40g
C%=8/32 x 100=25%
*( phần này là chắc chắn đúng)*
V(dd)=40l( vì 40g=40l)
Cm= n/V=0,2/40=0,005M
( phần này không chắc)
d này là khối lượng riêng hay là trọng lượng riêng vậy bạn?

a.\(n_{NaOH}=\dfrac{8}{40}=0,2mol\)
\(V_{dd}=\dfrac{120}{1,2}=100ml=0,1l\)
\(C_{M_{NaOH}}=\dfrac{0,2}{0,1}=2M\)
b.\(n_{NaOH}=\dfrac{21,6}{40}=0,54mol\)
\(V_{dd}=\dfrac{180}{1,2}=150ml=0,15l\)
\(C_{M_{NaOH}}=\dfrac{0,54}{0,15}=3,6M\)

\(n_{Na_2CO_3}=\dfrac{10,6}{106}=0,1\left(mol\right)\\ \rightarrow C_{M\left(Na_2CO_3\right)}=\dfrac{0,1}{0,2}=0,5M\)
Ta có: \(C\%=\dfrac{C_M.M}{10.D}\)
\(\rightarrow C\%=\dfrac{0,5.106}{10.1,05}=5,05\%\)

Ta có: \(m_{dd}=300\cdot1,05=315\left(g\right)\) \(\Rightarrow C\%_{Na_2CO_3}=\dfrac{15,9}{315}\cdot100\%\approx5,05\%\)
Mặt khác: \(n_{Na_2CO_3}=\dfrac{15,9}{106}=0,15\left(mol\right)\) \(\Rightarrow C_{M_{Na_2CO_3}}=\dfrac{0,15}{0,3}=0,5\left(M\right)\)
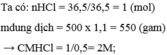
Câu 1 :
nNaOH = 20/40 = 0.5 (mol)
VddNaOH = 0.5/2 = 0.25 (l) = 250 (ml)
mdd NaOH = 250*1.05=262.5 (g)
C%NaOH = 20/262.5 *100% = 7.62%
Câu 2 :
nKOH = 20/56 = 0.357 (mol)
mddKOH = 20*100/5.6 = 357.1 (g)
Vdd KOH = 357.1/1.1 = 342.6 (ml) = 0.3426 (l)
CM KOH = 0.357/0.3426 = 1.1 (M)
Câu 3 :
mdd NaOH = 200 * 1.12 = 224 (g)
mNaOH = 224*40/100 = 89.6 (g)