Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

1.1. Al + NaOH + H2O ==> NaAlO2 + 3/2H2
nH2(1)=3,36/22,4=0.15(mol)
=> nAl(1)= nH2(1):3/2= 0.15:3/2= 0.1(mol)
2.Mg + 2HCl ==> MgCl2 + H2
3.2Al + 6HCl ==> 2AlCl3 + 3H2
4.Fe + 2HCl ==> FeCl2 + H2
=> \(n_{H_2\left(2,3,4\right)}=\) 10.08/22.4= 0.45(mol)
=> nH2(3)=0.1*3/2=0.15(mol)
MgCl2 + 2NaOH ==> Mg(OH)2 + 2NaCl
AlCl3 + 3NaOH ==> Al(OH)3 + 3NaCl
FeCl2 + 2NaOH ==> Fe(OH)2 + 2NaCl

CR ko tan là Cu
mCu= 12,8 (g)
\(\Rightarrow\) mMg + mFe = 23,6 - 12,8 = 10,8 (g)
Gọi nMg=x , nFe=y trong 10,8 g
\(\Rightarrow\) 24x + 56y = 10,8 (l)
Mg + 2HCl \(\rightarrow\) MgCl2 + H2
x ----> 2x (mol)
Fe + 2HCl \(\rightarrow\) FeCl2 + H2
y ----> 2y (mol)
nHCl = \(\frac{91,25.20\%}{36,5}\) = 0,5 (mol)
\(\Rightarrow\) 2x + 2y = 0,5 (ll)
Từ (l) và (ll) \(\Rightarrow\) \(\begin{cases}x=0,1\\y=0,15\end{cases}\)
mMg = 0,1 . 24 =2,4 (g)
mFe = 8,4 (g)

\(n_{H_2}=\dfrac{2,24}{22,4}=0,1(mol)\\ Mg+2HCl\to MgCl_2+H_2\\ MgO+2HCl\to MgCl_2+H_2O\\ \Rightarrow n_{Mg}=0,1(mol)\\ \Rightarrow \%_{Mg}=\dfrac{0,1.24}{6,4}.100\%=37,5\%\\ \Rightarrow \%_{MgO}=100\%-37,5\%=62,5\%\)
\(b,n_{MgO}=\dfrac{6,4-0,1.24}{40}=0,1(mol)\\ \Rightarrow n_{HCl}=2.0,1+2.0,1=0,4(mol)\\ \Rightarrow V_{dd_{HCl}}=\dfrac{0,4}{0,5}=0,8(l)\\ c,n_{MgCl_2}=0,1+0,1=0,2(mol)\\ \Rightarrow C_{M_{MgCl_2}}=\dfrac{0,2}{0,8}=0,25M\)

\(a,Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\\ n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\\ b,n_{Fe}=n_{H_2}=0,2\left(mol\right)\\ \%m_{Fe}=\dfrac{0,2.56}{12,8}.100\%=87,5\%\\ \%m_{Fe_2O_3}=100\%-87,5\%=12,5\%\\ c,n_{Fe_2O_3}=\dfrac{12,8-11,2}{160}=0,01\left(mol\right)\\ n_{H_2SO_4}=n_{Fe}+3n_{Fe_2O_3}=0,2+3.0,01=0,23\left(mol\right)\\ V_{ddH_2SO_4}=\dfrac{0,23}{0,46}=0,5\left(M\right)\)

Câu 1:
ta có pthh
2Al + 6HCl \(\rightarrow\)2 AlCl3 + 3H2 (1)
Zn + 2HCl \(\rightarrow\) ZnCl2 + H2 (2)
a, Theo đề bài ta có
nHCl=CM.V = 2.0,3=0,6 mol
Gọi x là số mol của HCl tham gia vào pthh 1
số mol của HCl tham gia vào pthh 2 là 0,6-x mol
Theo pthh 1 và 2 ta có
nAl=2/6nHCl=2/6x mol
nZn=1/2nHCl=1/2(0,6-x) mol
Theo đề bài ta có hệ pt
27.2/6x +65.1/2(0,6-x) = 12,45
\(\Leftrightarrow\) 9x + 19,5 - 32,5x = 12,45
\(\Leftrightarrow\) -23,5x=-7,05
-> x=0,3 mol
-> nAl=2/6nHCl=2/6.0,3=0,1 mol
nZn=1/2nHCl=1/2(0,6-0,3)=0,15 mol
-> %mAl=\(\dfrac{\left(0,1.27\right).100\%}{12,45}\approx21,69\%\)
%mZn=100%-21,6%=78,31%
b, Cách 1:
Theo pthh 1 và 2
nH2=1/2nHCl=1/2.0,6=0,3 mol
-> VH2=0,3.22,4=6,72 (l)
Cách 2
Theo pthh 1
nH2=1/2nHCl=1/2.0,3=0,15 mol
Theo pthh 2
nH2=nZn=1/2nHCl=1/2.0,3=0,15 mol
-> VH2(đktc)=(0,15+0,15).22,4=6,72(l)
ĐAY LÀ BÀI HÓA 8 Mà SAO ĐĂNG LÊN HÓA (9
Câu 2:
Ta có pthh
(1) Fe2O3 + 3H2SO4 \(\rightarrow\) Fe2(SO4)3 + 3H2O
(2) ZnO + H2SO4 \(\rightarrow\) ZnSO4 + H2O
a, Theo đề bài ta có
nH2SO4=CM.V=2.0,15=0,3 mol
Gọi x là số mol của H2SO4 tham gia vào pthh 1
Số mol của H2SO4 tham gia vào pthh 2 là 0,3 - x mol
Theo pth 1 và 2
nFe2O3=1/3nH2SO4=1/3x mol
nZnO = nH2SO4 = (0,3-x ) mol
Theo đề bài ta có hệ pt
160.1/3x + 81(0,3-x) = 20,15
\(\Leftrightarrow\) 53,33x + 24,3 - 81x = 20,15
\(\Leftrightarrow\) -27,67x = -4,15
-> x\(\approx\)0,15 mol
-> nFe2O3=1/3nH2SO4=1/3.0,15=0,05 mol
-> mFe2O3=0,05.160=8 g
mZnO=20,15-8=12,15 g
b, Theo pthh 1 và 2
nFe2(SO4)3=nFeO=0,05 mol
nZnSO4=nZnO=0,15 mol
-> CM\(_{Fe2\left(SO4\right)3}=\dfrac{0,05}{0,15}\approx0,333M\)
CM\(_{ZnO}=\dfrac{0,15}{0,15}=1M\)
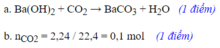
Câu 1:
Ca+2H2O\(\rightarrow\)Ca(OH)2+H2
CaO+H2O\(\rightarrow\)Ca(OH)2
\(n_{Ca}=n_{H_2}=\dfrac{V}{22,4}=\dfrac{4,48}{22,4}=0,2mol\)
mCa=0,2.40=8 gam
%Ca=\(\dfrac{8.100}{20}=40\%\)