Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Câu 1 :
\(2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ 2Mg + O_2 \xrightarrow{t^o} 2MgO\\ 2Cu + O_2 \xrightarrow{t^o} 2CuO\\ S + O_2 \xrightarrow{t^o} SO_2\\ 4Al + 3O_2 \xrightarrow{t^o} 2Al_2O_3\\ C + O_2 \xrightarrow{t^o} CO_2\\ 4P + 5O_2 \xrightarrow{t^o} 2P_2O_5\)

a)
\(C + O_2 \xrightarrow{t^o} CO_2\\ n_{CO_2} = n_{O_2} = \dfrac{6,4}{32} = 0,2(mol)\\ \Rightarrow m_{CO_2} = 0,2.44 = 8,8(gam)\)
b)
\(n_C = \dfrac{6}{12} = 0,5(mol)\\ n_{O_2} =\dfrac{19,2}{32} = 0,6(mol)\\ C + O_2 \xrightarrow{t^o} CO_2\)
\(n_C = 0,5 < n_{O_2} = 0,6 \Rightarrow\) Oxi dư.
\(n_{CO_2} = n_C = 0,5(mol)\\ \Rightarrow m_{CO_2} = 0,5.44 = 22(gam)\)

bài 5:
PTHH: C + O2 -> CO2
a) Số Mol của Oxi là:
ADCT: n= m/M
=>nO2= 6,4/ 32= 0,2 ( mol)
theo PT: nCO2 = nO2 = 0,2 mol
klg của CO2 là:
ADCT: m = n. M
=> mCO2= 0.2 . 12 = 2,4 (g)

\(C+O_2\underrightarrow{^{to}}CO_2\)
a) \(n_{O2}=\frac{6,4}{32}=0,2\left(mol\right)\)
\(\Rightarrow n_{CO2}=n_{O2}=0,2\left(mol\right)\)
\(\Rightarrow m_{CO2}=0,2.22,4=8,8\left(g\right)\)
b) \(n_C=\frac{0,3}{12}=0,025\left(mol\right)\)
\(\Rightarrow n_{CO2}=n_C=0,025\left(mol\right)\)
\(\Rightarrow m_{CO2}=0,025.44=1,1\left(g\right)\)
c) \(n_C=0,3\left(mol\right)\)
\(n_{O2}=0,2\left(mol\right)\)
Nên C dư \(\rightarrow\) nCO2 tính theo nO2
\(n_{CO2}=0,2\left(mol\right)\)
\(\Rightarrow m_{CO2}=0,2.44=8,8\left(g\right)\)
d) \(n_C=\frac{6}{12}=0,5\left(mol\right)\)
\(n_{O2}=\frac{19,2}{32}=0,6\left(mol\right)\)
Nên O2 dư
nCO2 được tính theo nC
\(n_{CO2}=0,5\left(mol\right)\)
\(\Rightarrow m_{CO2}=0,5.44=22\left(g\right)\)

1) \(C+O_2\rightarrow CO_2\\
C+CO_2\rightarrow2CO\)
2)
\(pthh:C+O_2\rightarrow CO_2\)
=> số mol bằng nhau
\(n_{O_2}=\dfrac{6,4}{16}=0,4\left(mol\right)\)
áp vào pt trên ta có : nCO2 = 0,4 (mol)
=> \(m_{CO_2}=0,4.44=17,6\left(g\right)\)
=> dCO2/H2 = 44/2 = 22
dCO2/H2 = 44/2 = 22

\(1,2H_2+O_2\underrightarrow{t}2H_2O\)
\(2Mg+O_2\underrightarrow{t}2MgO\)
\(2Cu+O_2\underrightarrow{t}2CuO\)
\(S+O_2\underrightarrow{t}SO_2\)
\(4Al+3O_2\underrightarrow{t}2Al_2O_3\)
\(C+O_2\underrightarrow{t}CO_2\)
\(4P+5O_2\underrightarrow{t}2P_2O_5\)
\(2,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(a,n_{O_2}=0,2\left(mol\right)\Rightarrow n_{CO_2}=0,2\left(mol\right)\Rightarrow m_{CO_2}=8,8\left(g\right)\)
\(b,n_C=0,3\left(mol\right)\Rightarrow n_{CO_2}=0,3\left(mol\right)\Rightarrow m_{CO_2}=13,2\left(g\right)\)
c, Vì\(\frac{0,3}{1}>\frac{0,2}{1}\)nên C phản ửng dư, O2 phản ứng hết, Bài toán tính theo O2
\(n_{O_2}=0,2\left(mol\right)\Rightarrow n_{CO_2}=0,2\left(mol\right)\Rightarrow m_{CO_2}=8,8\left(g\right)\)
\(3,PTHH:CH_4+2O_2\underrightarrow{t}CO_2+2H_2O\)
\(C_2H_2+\frac{5}{2}O_2\underrightarrow{t}2CO_2+H_2O\)
\(C_2H_6O+3O_2\underrightarrow{t}2CO_2+3H_2O\)
\(4,a,PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
\(n_P=1,5\left(mol\right)\Rightarrow n_{O_2}=1,2\left(mol\right)\Rightarrow m_{O_2}=38,4\left(g\right)\)
\(b,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(n_C=2,5\left(mol\right)\Rightarrow n_{O_2}=2,5\left(mol\right)\Rightarrow m_{O_2}=80\left(g\right)\)
\(c,PTHH:4Al+3O_2\underrightarrow{t}2Al_2O_3\)
\(n_{Al}=2,5\left(mol\right)\Rightarrow n_{O_2}=1,875\left(mol\right)\Rightarrow m_{O_2}=60\left(g\right)\)
\(d,PTHH:2H_2+O_2\underrightarrow{t}2H_2O\)
\(TH_1:\left(đktc\right)n_{H_2}=1,5\left(mol\right)\Rightarrow n_{O_2}=0,75\left(mol\right)\Rightarrow m_{O_2}=24\left(g\right)\)
\(TH_2:\left(đkt\right)n_{H_2}=1,4\left(mol\right)\Rightarrow n_{O_2}=0,7\left(mol\right)\Rightarrow m_{O_2}=22,4\left(g\right)\)
\(5,PTHH:S+O_2\underrightarrow{t}SO_2\)
\(n_{O_2}=0,46875\left(mol\right)\)
\(n_{SO_2}=0,3\left(mol\right)\)
Vì\(0,46875>0,3\left(n_{O_2}>n_{SO_2}\right)\)nên S phản ứng hết, bài toán tính theo S.
\(a,\Rightarrow n_S=n_{SO_2}=0,3\left(mol\right)\Rightarrow m_S=9,6\left(g\right)\)
\(n_{O_2}\left(dư\right)=0,16875\left(mol\right)\Rightarrow m_{O_2}\left(dư\right)=5,4\left(g\right)\)
\(6,a,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_C=1,5\left(mol\right)\Rightarrow m_C=18\left(g\right)\)
\(b,PTHH:2H_2+O_2\underrightarrow{t}2H_2O\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_{H_2}=0,75\left(mol\right)\Rightarrow m_{H_2}=1,5\left(g\right)\)
\(c,PTHH:S+O_2\underrightarrow{t}SO_2\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_S=1,5\left(mol\right)\Rightarrow m_S=48\left(g\right)\)
\(d,PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_P=1,2\left(mol\right)\Rightarrow m_P=37,2\left(g\right)\)
\(7,n_{O_2}=5\left(mol\right)\Rightarrow V_{O_2}=112\left(l\right)\left(đktc\right)\);\(V_{O_2}=120\left(l\right)\left(đkt\right)\)
\(8,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(m_C=0,96\left(kg\right)\Rightarrow n_C=0,08\left(kmol\right)=80\left(mol\right)\Rightarrow n_{O_2}=80\left(mol\right)\Rightarrow V_{O_2}=1792\left(l\right)\)
\(9,n_p=0,2\left(mol\right);n_{O_2}=0,3\left(mol\right)\)
\(PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
Vì\(\frac{0,2}{4}< \frac{0,3}{5}\)nên P hết O2 dư, bài toán tính theo P.
\(a,n_{O_2}\left(dư\right)=0,05\left(mol\right)\Rightarrow m_{O_2}\left(dư\right)=1,6\left(g\right)\)
\(b,n_{P_2O_5}=0,1\left(mol\right)\Rightarrow m_{P_2O_5}=14,2\left(g\right)\)

\(a,\text {Bảo toàn KL: }m_{C}+m_{O_2}=m_{CO_2}\\ \Rightarrow m_{CO_2}=m_{C}+m_{O_2}=16+6=22(g)\\ b,m_{C}=m_{CO_2}-m_{O_2}=44-32=12(g)\)
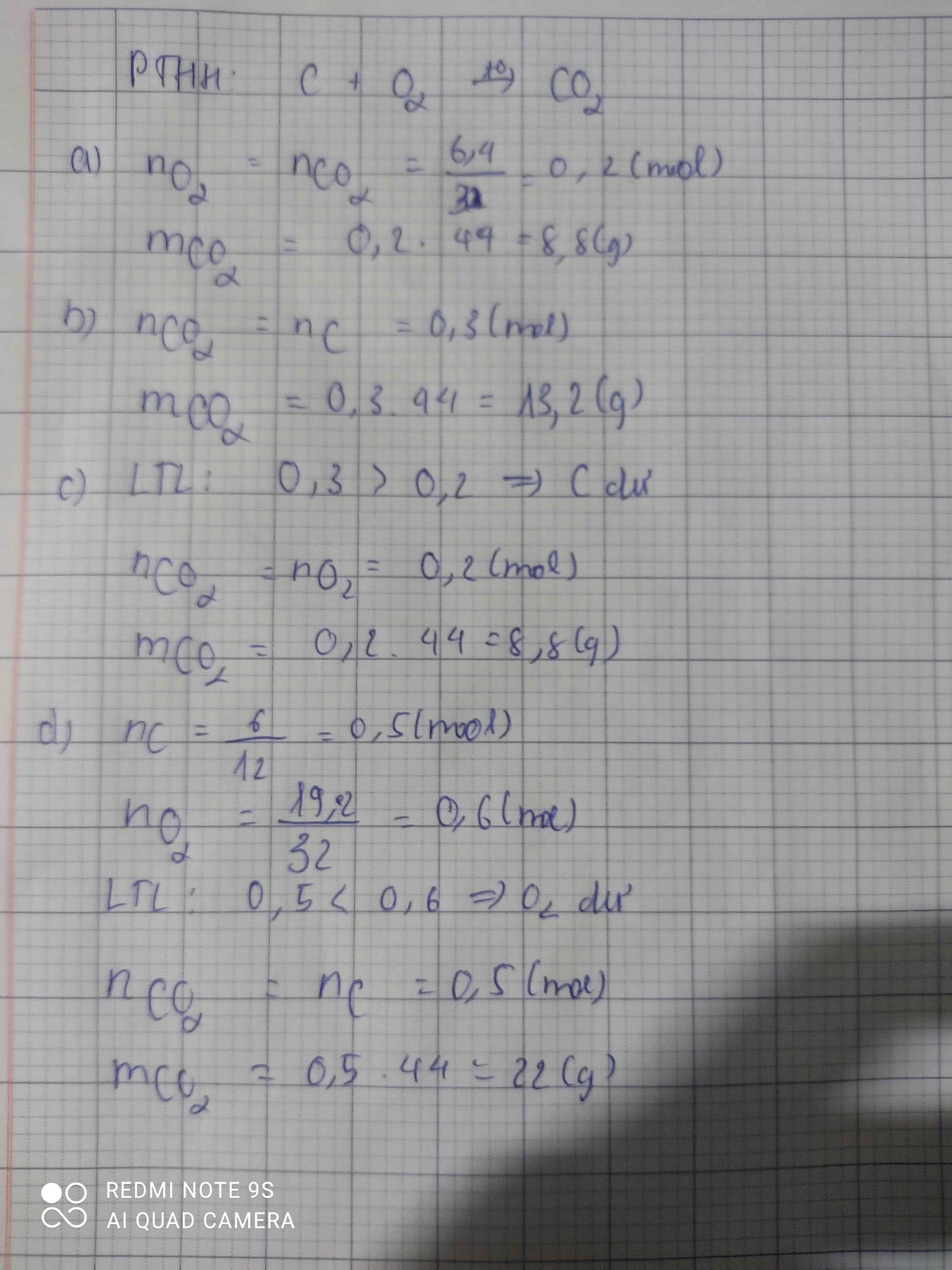
C + O2 -> CO2 (1)
a;
Từ 1:
nC=nCO2=0,2(mol)
mCO2=44.0,2=8,8(g)
b;
nC=0,3(mol)
Từ 1:
nC=nCO2=0,3(mol)
mCO2=44.0,3=13,2(g)
c;
nO2=0,2(mol)
Từ 1:
nO2=nCO2=0,2(mol)
mCO2=44.0,2=8,8(g)
a) \(C+O_2\underrightarrow{t^o}CO_2\)
\(m_{CO_2}=0,2\cdot44=8,8\left(g\right)\)
b)\(n_{CO_2}=\dfrac{3,6}{12}=0,3\left(mol\right)\\ C+O_2\underrightarrow{t^o}CO_2\\ m_{CO_2}=0,3\cdot44=13,2\left(g\right)\)
c)\(n_{O_2}=\dfrac{6,4}{32}=0,2\left(mol\right)\\ C+O_2\underrightarrow{t^o}CO_2\\ m_{CO_2}=0,2\cdot44=8,8\left(g\right)\)