Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PT: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
a, Ta có: \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{30}{98}=\dfrac{15}{49}\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,2}{2}< \dfrac{\dfrac{15}{49}}{3}\) , ta được H2SO4 dư.
b, Theo PT: \(n_{H_2}=\dfrac{3}{2}n_{Al}=0,3\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,3.22,4=6,72\left(l\right)\)
c, Sau phản ứng, trong cốc có H2SO4 dư và Al2(SO4)3.
Theo PT: \(\left\{{}\begin{matrix}n_{H_2SO_4\left(pư\right)}=\dfrac{3}{2}n_{Al}=0,3\left(mol\right)\\n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{H_2SO_4\left(dư\right)}=\dfrac{15}{49}-0,3\approx0,006\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{H_2SO_4\left(dư\right)}=0,006.98=0,588\left(g\right)\\m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2\left(g\right)\end{matrix}\right.\)
Bạn tham khảo nhé!

2Al+3H2SO4->Al2(SO4)3+3H2
0,2-----0,3-------0,1------------0,3
n Al=\(\dfrac{5,4}{27}\)=0,2 mol
n H2SO4= \(\dfrac{30}{98}\)=0,306 mol
=>H2SO4 còn dư
=>VH2=0,3.22,4=6,72l
=>m Al2(SO4)3=0,1.342=34,2g
=>m H2SO4 dư=0,006.98=0,588g
\(n_{Al}=\dfrac{m_{Al}}{M_{Al}}=\dfrac{5,4}{27}=0,2mol\)
\(n_{H_2SO_4}=\dfrac{m_{H_2SO_4}}{M_{H_2SO_4}}=\dfrac{30}{98}=\dfrac{15}{49}mol\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
2 3 1 3 ( mol )
0,2 15/49 ( mol )
Ta có: \(\dfrac{0,2}{2}< \dfrac{15}{49}:3\)
=> Chất còn dư là \(H_2SO_4\)
\(V_{H_2}=n_{H_2}.22,4=\left(\dfrac{0,2.3}{2}\right).22,4=6,72l\)
\(m_{Al_2\left(SO_4\right)_3}=n_{Al_2\left(SO_4\right)_3}.M_{Al_2\left(SO_4\right)_3}=\left(\dfrac{0,2.1}{2}\right).342=34,2g\)
\(m_{H_2SO_4\left(du\right)}=n_{H_2SO_4\left(du\right)}.M_{H_2SO_4}=\left(\dfrac{15}{49}-\dfrac{0,2.3}{2}\right).98=0,6g\)

\(n_{Al}=\dfrac{2,5}{27}=\dfrac{25}{270}=\dfrac{5}{54}\left(mol\right)\\ n_{H_2SO_4}=0,5\left(mol\right)\\ a,2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ b,Vì:\dfrac{\dfrac{5}{54}}{2}< \dfrac{0,5}{4}\Rightarrow H_2SO_4dư\\ b,n_{H_2SO_4\left(dư\right)}=0,5-\dfrac{3}{2}.\dfrac{5}{54}=\dfrac{13}{36}\left(mol\right)\\ \Rightarrow m_{H_2SO_4}=\dfrac{13}{36}.98=\dfrac{637}{18}\left(g\right)\\ c,n_{H_2}=\dfrac{3}{2}.n_{Al}=\dfrac{3}{2}.\dfrac{5}{54}=\dfrac{5}{36}\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=\dfrac{5}{36}.22,4=\dfrac{28}{9}\left(l\right)\)

a: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
b: \(n_{Al}=\dfrac{2.5}{27}< \dfrac{1}{4}\)
=>H2SO4 dư, Al đủ
\(m_{H_2SO_4}=0.25\cdot98=24.5\left(g\right)\)
c: \(n_{Al_2\left(SO_4\right)_3}=\dfrac{2.5}{54}=\dfrac{5}{108}\left(mol\right)\)
\(\Leftrightarrow n_{H_2}=\dfrac{5}{36}\left(mol\right)\)
\(V_{H_2}=\dfrac{5}{36}\cdot22.4=\dfrac{28}{9}\left(lít\right)\)
Mình thấy bạn Thịnh tính lượng dư sai
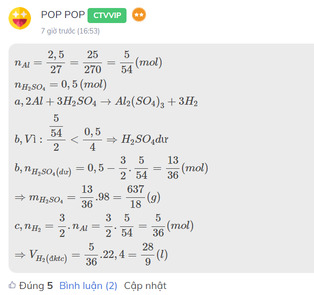
Đây là bài mình từng làm, bạn tham khảo nhé!

PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\)
Làm gộp các phần còn lại
Ta có: \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\) \(\Rightarrow\left\{{}\begin{matrix}n_{Al_2\left(SO_4\right)_3}=0,1mol\\n_{H_2SO_4}=n_{H_2}=0,3mol\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}V_{H_2}=0,3\cdot22,4=6,72\left(l\right)\\m_{Al_2\left(SO_4\right)_3}=0,1\cdot342=34,2\left(g\right)\\m_{H_2SO_4}=0,3\cdot98=29,4\left(g\right)\end{matrix}\right.\)

a.b.\(n_{Al}=\dfrac{5,4}{27}=0,2mol\)
\(n_{H_2SO_4}=\dfrac{39,2}{98}=0,4mol\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
Xét: \(\dfrac{0,2}{2}\) < \(\dfrac{0,4}{3}\) ( mol )
0,2 0,3 0,1 0,3 ( mol )
\(m_{H_2SO_4\left(dư\right)}=\left(0,4-0,3\right).98=9,8g\)
\(m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2g\)
c.\(2H_2+O_2\rightarrow\left(t^o\right)2H_2O\)
0,3 0,15 ( mol )
\(V_{kk}=V_{O_2}.5=\left(0,15.22,4\right).5=16,8l\)

\(n_{Al}=\dfrac{5.4}{27}=0.2\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{49}{98}=0.5\left(mol\right)\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(0.2........0.3.................................0.3\)
\(m_{H_2SO_4\left(dư\right)}=\left(0.5-0.3\right)\cdot98=19.6\left(g\right)\)
\(V_{H_2}=0.3\cdot22.4=6.72\left(l\right)\)

\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
\(n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
\(pthh:Zn+2HCl->ZnCl_2+H_2\)
LTL :
\(\dfrac{0,2}{1}=\dfrac{0,4}{2}\)
=> ko chất nào dư
theo pthh : \(n_{H_2}=n_{Zn}=0,2\left(mol\right)\\
=>V_{H_2}=0,2.22,4=4,48\left(l\right)\)
Bài 8 (bài 7 bạn ở trên làm rồi)
\(n_{H_2\left(Al\right)}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ n_{H_2\left(Zn\right)}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PTHH:
2Al + 3H2SO4 ---> Al2(SO4)3 + 3H2
0,2 0,3 0,1 0,3
Zn + H2SO4 ---> ZnSO4 + H2
0,3 0,3 0,3 0,3
\(a,\left\{{}\begin{matrix}m_{Al}=0,3.27=8,1\left(g\right)\\m_{Zn}=0,3.65=19,5\left(g\right)\end{matrix}\right.\\ b,m_{H_2SO_4}=\left(0,3+0,3\right).98=58,8\left(g\right)\)
c, Hợp chất tạo thành thuộc loại muối trung hoà
mmuôí = 0,1.342 + 0,3.161 = 82,5 (g)
Thanks ạ