Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{6.72}{22.4}=0.3\left(mol\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(.........0.6............0.3\)
\(C_{M_{HCl}}=\dfrac{0.6}{0.3}=2\left(M\right)\)
\(n_{Fe_2O_3}=\dfrac{48}{160}=0.3\left(mol\right)\)
\(Fe_2O_3+3H_2\underrightarrow{^{t^0}}2Fe+3H_2O\)
\(1..............3\)
\(0.3..........0.3\)
\(LTL:\dfrac{0.3}{1}>\dfrac{0.3}{3}\Rightarrow Fe_2O_3dư\)
\(n_{Fe}=\dfrac{2}{3}n_{H_2}=\dfrac{2}{3}\cdot0.3=0.2\left(mol\right)\)
\(m_{Fe}=0.2\cdot56=11.2\left(g\right)\)

\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\\a, PTHH:Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ b,n_{H_2}=n_{H_2SO_4}=n_{Fe}=0,2\left(mol\right)\\ V_{H_2\left(đkc\right)}=0,2.24,79=4,958\left(l\right)\\ c,C_{MddH_2SO_4}=\dfrac{0,2}{0,4}=0,5\left(M\right)\)

a, Ta có: \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
___0,1_________________0,1 (mol)
Ta có: \(V_{H_2}=0,1.22,4=2,24\left(l\right)\)
b, PT: \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
Theo PT: \(n_{Fe}=\dfrac{2}{3}n_{H_2}=\dfrac{1}{15}\left(mol\right)\)
\(\Rightarrow m_{Fe}=\dfrac{1}{15}.56\approx3,73\left(g\right)\)
Bạn tham khảo nhé!

a, \(Fe_3O_4+4H_2\underrightarrow{t^o}3Fe+4H_2O\)
b, \(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
Theo PT: \(n_{Fe_3O_4}=\dfrac{1}{3}n_{Fe}=\dfrac{1}{15}\left(mol\right)\Rightarrow m_{Fe_3O_4}=\dfrac{1}{15}.232=\dfrac{232}{15}\left(g\right)\)
c, \(n_{H_2}=\dfrac{4}{3}n_{Fe}=\dfrac{4}{15}\left(mol\right)\Rightarrow V_{H_2}=\dfrac{4}{15}.22,4=\dfrac{448}{75}\left(l\right)\)
d, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(n_{Zn}=n_{H_2}=\dfrac{4}{15}\left(mol\right)\Rightarrow m_{Zn}=\dfrac{4}{15}.65=\dfrac{52}{3}\left(g\right)\)
\(n_{HCl}=2n_{H_2}=\dfrac{8}{15}\left(mol\right)\Rightarrow m_{HCl}=\dfrac{8}{15}.36,5=\dfrac{292}{15}\left(g\right)\)

a, PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, Ta có: \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}=0,1\left(mol\right)\Rightarrow V_{H_2}=0,1.22,4=2,24\left(l\right)\)
c, PT: \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
Theo PT: \(n_{Fe}=\dfrac{2}{3}n_{H_2}=\dfrac{1}{15}\left(mol\right)\Rightarrow m_{Fe}=\dfrac{1}{15}.56=\dfrac{56}{15}\left(g\right)\)

nZn = 6.5/65 = 0.1 (mol)
Zn + 2HCl => ZnCl2 + H2
0.1.......0.2...................0.1
VddHCl = 0.2/2 = 0.1 (l)
nFe = 3/56 (mol)
Fe2O3 + 3H2 -to-> 2Fe + 3H2O
.................9/112........3/56
H% = 9/112 / 0.1 * 100% = 80.35%
a) Zn + 2HCl $\to$ ZnCl2 + H2
b) n Zn = 6,5/65 = 0,1(mol)
Theo PTHH : n HCl = 2n Zn = 0,2(mol)
=> V dd HCl = 0,2/2 = 0,1(lít)
c) n Fe = 3/56 (mol)
Fe2O3 + 3H2 $\xrightarrow{t^o}$ 2Fe + 3H2O
Theo PTHH :
n H2 = 3/2 n Fe = 9/112(mol)
Vậy :
H = $\dfrac{ \dfrac{9}{112} }{0,1}$ .100% = 80,36%

Zn+2Hcl->ZnCl2+H2
0,2---0,4----0,2----0,2
n Zn=0,2 mol
=>VH2 =0,2.22,4=4,48l
mZncl2=0,2.136=27,2g
3H2+Fe2O3-to>2Fe+3H2O
0,2---------------------2\15
->m Fe=2\15.56=7,467g
nZn= 13/65=0,2(mol)
a) PTHH: Zn + 2 HCl -> ZnCl2 + H2
b) nH2=nZnCl2=nZn=0,2(mol)
=>V(H2,đktc)=0,2 x 22,4= 4,48(l)
c) khối lượng muối sau phản ứng chứ nhỉ?
mZnCl2=136.0,2=27,2(g)
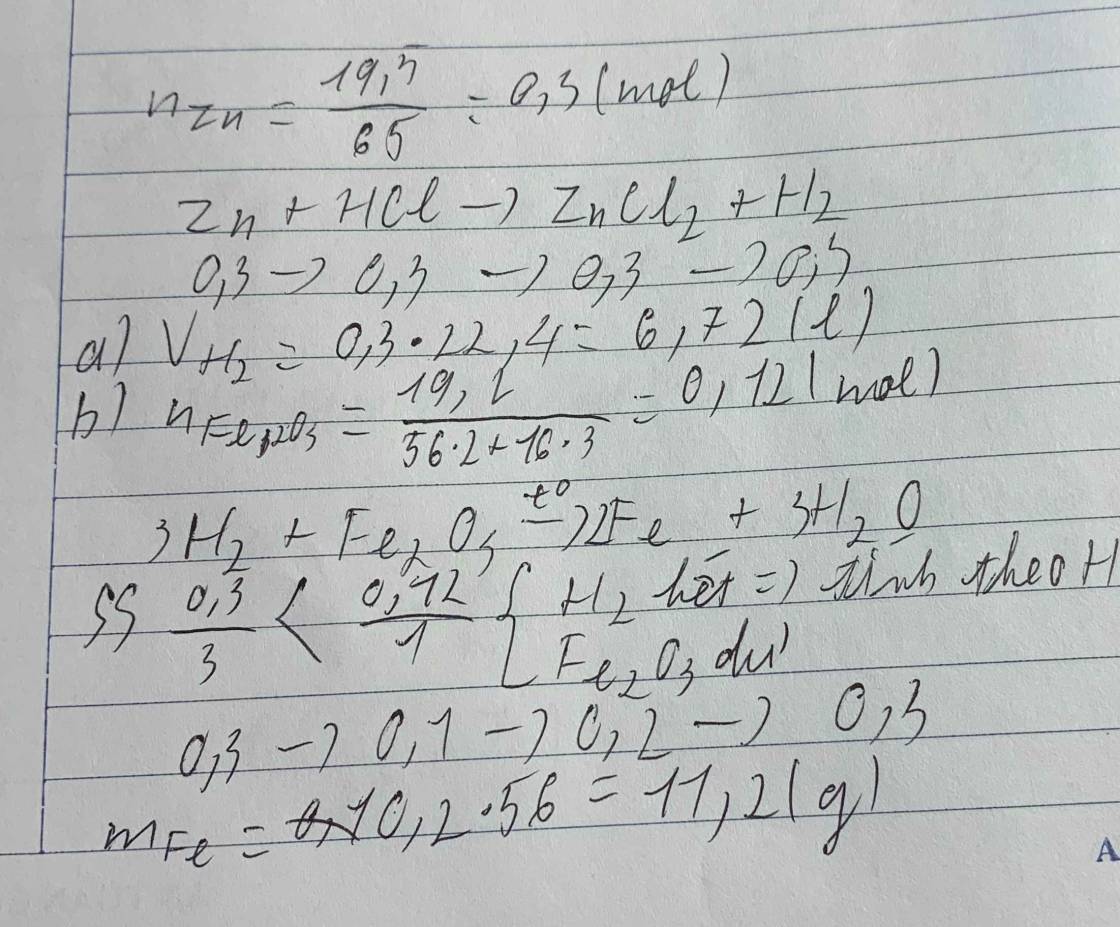
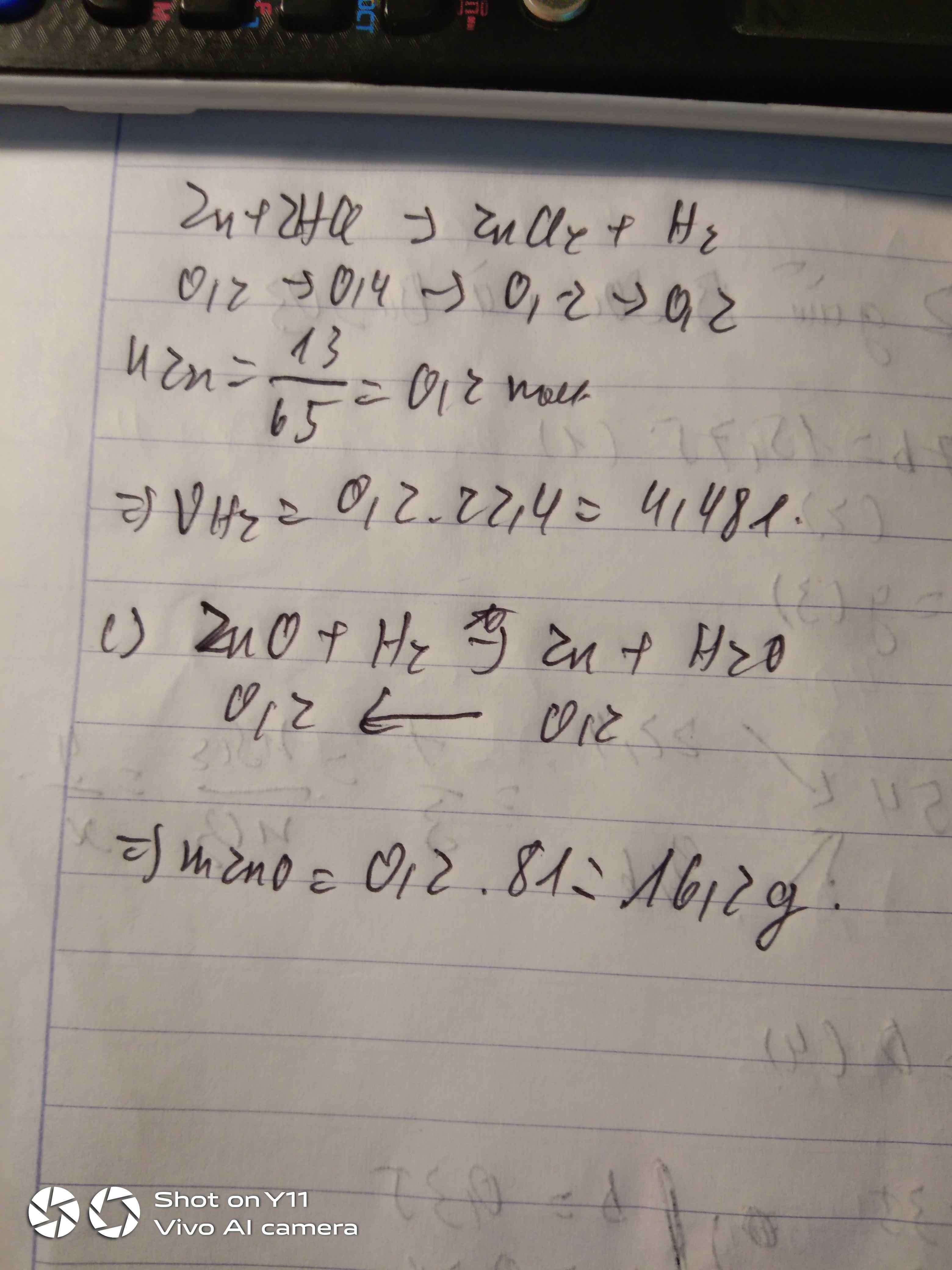
Bài 3:
\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
a, PTHH: Fe3O4 + 4H2 --to--> 3Fe + 4H2O
0,1<------0,4
Zn + 2HCl ---> ZnCl2 + H2
0,4<-------------------------0,4
b, mFe3O4 = 0,1.232 = 23,2 (g)
c, mZn = 0,4.65 = 26 (g)
Bài 4:
\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
a, PTHH: Zn + 2HCl ---> ZnCl2 + H2
0,1---->0,2---------------->0,1
b, VH2 = 0,1.22,4 = 2,24 (l)
c, \(C_{M\left(HCl\right)}=\dfrac{0,2}{0,2}=1M\)