Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{KClO_3}=\dfrac{29.4}{122.5}=0.24\left(mol\right)\)
\(2KClO_3\underrightarrow{^{^{t^0}}}2KCl+3O_2\)
\(0.24.....................0.36\)
KClO3 : Kali clorat
KCl : Kali clorua
\(V_{O_2}=0.36\cdot22.4=8.064\left(l\right)\)
\(b.\)
\(n_P=\dfrac{6.2}{31}=0.2\left(mol\right)\)
\(4P+5O_2\underrightarrow{^{^{t^0}}}2P_2O_5\)
Lập tỉ lệ :
\(\dfrac{0.2}{4}< \dfrac{0.36}{5}\) => O2 dư
\(n_{O_2\left(dư\right)}=0.36-0.2\cdot\dfrac{5}{4}=0.11\left(mol\right)\)
\(m_{O_2}=0.11\cdot32=3.52\left(g\right)\)
\(m_{P_2O_5}=0.1\cdot142=14.2\left(g\right)\)
Chúc em học tốt nhé !

nP= 0,2(mol)
a) PTHH: 4P + 5 O2 -to-> 2 P2O5
0,2_________0,25_____0,1(mol)
b) V(O2,đktc)=0,25 x 22,4= 5,6(l)
c) mP2O5=142 x 0,1=14,2(g)

\(n_{O_2\left(đktc\right)}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ 4P+5O_2\underrightarrow{^{to}}2P_2O_5\\ 0,12........0,15.........0,06\left(mol\right)\\ m_P=0,12.31=3,72\left(g\right)\)

$\rm n_P=\dfrac{6,2}{31}=0,2(mol)$
$\rm a)PTHH:4P+5O_2\xrightarrow{t^o}2P_2O_5$
$\rm b)$ Theo PT: $\rm n_{O_2}=1,25n_P=0,25(mol)$
$\rm V_{O_2}=0,25.22,4=5,6(lít)$
a, Số mol P là
n = m/M = 6,2/31 = 0,2 (mol)
a, PTHH : 4P + 5O2 -t0> 2P2O5
4 5 2
0,2 mol -> 0,25 mol 0,1 mol
b, Thể tích O2 theo đktc là :
V = n . 22,4 = 0,25 . 22,4 = 5,6 (l)

a) 4P + 5O2 --to--> 2P2O5
b) \(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\)
PTHH: 4P + 5O2 --to--> 2P2O5
0,2-->0,25------->0,1
=> mP2O5 = 0,1.142 = 14,2(g)
c) VO2 = 0,25.22,4 = 5,6(l)

a, \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
b, \(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{5}{4}n_P=0,25\left(mol\right)\Rightarrow V_{O_2}=0,25.22,4=5,6\left(l\right)\)
c, \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
Theo PT: \(n_{KMnO_4}=2n_{O_2}=0,5\left(mol\right)\Rightarrow m_{KMnO_4}=0,5.158=79\left(g\right)\)

4P+5O2→2P2O5
+nP=\(\dfrac{6,2}{31}\)=0,2(mol)
+nP2O5=\(\dfrac{1}{2}\)nP=0,1(mol)
+mP2O5=0,1.142=14,2(gam)
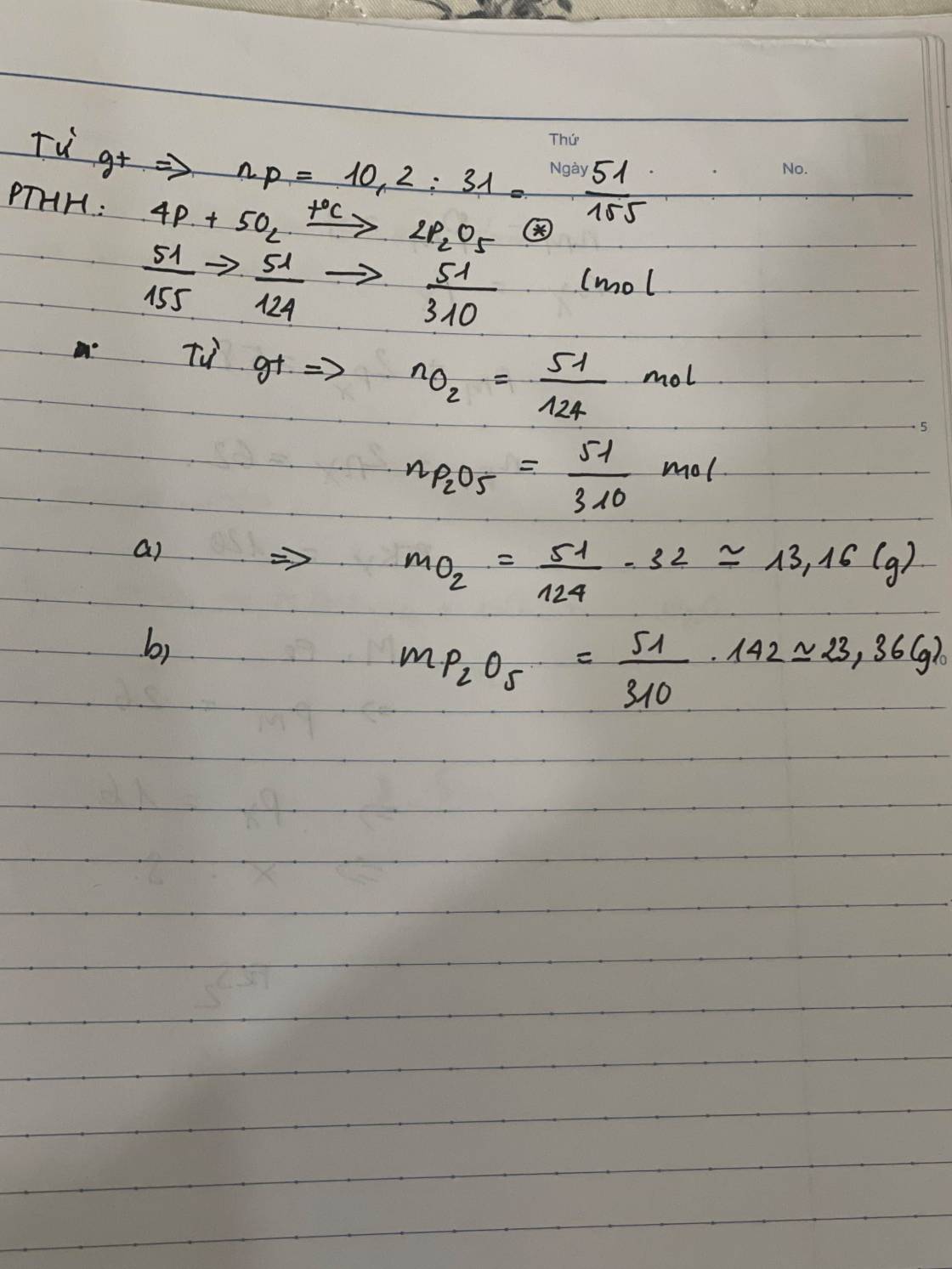
\(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\)
PTHH : 4P + 5O2 -> 2P2O5
=> \(n_{P_2O_5}=\dfrac{1}{2}n_P=0,1\left(mol\right)\)
=> \(m_{P_2O_5}=0,1.142=14,2\left(g\right)\)
Theo ĐLBTKL
\(m_P+m_{O_2}=m_{P_2O_5}\\ =>m_{O_2}=14,2-6,2=8\left(g\right)\)
=> \(n_{O_2}=\dfrac{8}{32}=0,25\left(mol\right)\\ V_{O_2}=0,25.22,4=5,6\left(l\right)\)
3,584l